Perch, Nile (Lates Niloticus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Climate Change at Lake Victoria
East Africa Living Lakes Network, C/O OSIENALA (Friends of Lake Victoria). Dunga Beach Kisumu Ann Nabangala Obae. Coordinator. Phone: +254-20-3588681. Email: [email protected] Background It is the Second largest fresh water lake in the World and it is surrounded by three East African States: With 6% in Kenya; Tanzania 52% and Uganda 42%. It is Located at 0:21 0 North and 3:00 0 South of the Equator Lake Victoria has a total length of 3,440 kms and 240 kms wide from East to West and is 1,134 meters above sea level with maximum depth of 82m.Its surface area is 68,870 km 2, catchment area of 180,950 km 2 Generally shallow with maximum depth of 84 meters and mean depth of 40 meters Average inflows and out flows of Lake Victoria Type of flow Flow (m3/s) Percentage (%) Inflows Rain over Lake 3,631 82 Basin Discharge 778 18 Type of flow Flow ( m3/s) Percentage (%) Out flows Evaporation from lake -3,300 76 Nile River - 1,046 24 Balance +33 Sources of Lake Victoria from Kenyan water towers Sondu Miriu river Yala river Nzoia river Mara River Kuja river Impacts and effects Changes in water budget are respectively accompanied by water level fluctuation and promote thermal structures which result in nutrient and food web dynamics. Studies have proved that there is a positive correlation between water level and fish landings (Williams, l972) The abundant fish catches are highly correlated with rainfall and lake levels. The records show that the catches reduced to between 60% and 70% during the current reduction of water level in Nyanza Gulf. -

Fishing Gear in the Sondu-Miriu River: Level of Use, Preference and Selectivity Waswala-Olewe M
Fishing Gear in the Sondu-Miriu River: Level of Use, Preference and Selectivity Waswala-Olewe M. Brian, Okot-Okumu James and Abila O. Richard Waswala- Olewe M. Brian Okumu James Abila o. Richard Abstract: Artisan fishers of Osodo beach of Sondu-Miriu River (Kenya) use both traditional and modern gear to catch riverine fish species. This study, conducted between August 2006 and July 2007, revealed that fishers most predominantly used gear were the seine nets (42%) and the gill nets (28%). Other used gear include long lines (14%); fish baskets (9%) and weirs (7%). The selectivity of this fishing gear varied with the developmental stages of the fish to be caught. Non- selective gear caught both targeted and non-targeted species irrespective of size and development stages. The ranking of selective to non-selective fishing gear was the long lines, fish baskets, weirs, gill nets and beach nets at 2%, 11%, 16%, 24% and 32%, respectively. The non-selective fishing gear may have negative impacts on the riverine fish by reducing spawning biomass and lacustrine fish recruitment. These findings underscore the need for greater appreciation, research, and adaptation of appropriate fishing gear to ensure sustainable utilization of the riverine fisheries in Sondu-Miriu River. Key words: Sondu-Miriu River, Osodo beach, riverine fish, lacustrine fish, fishing gear Introduction Nile Perch (Lates nilotica) (Ogutu-Ohwayo & Balirwa ish plays an important role in both the aquatic 2006). Fecosystem and to the human population. They are Rivers are crucial breeding and nursery grounds for good indicators of environmental health within rivers the riverine fish species which later repopulate the lakes. -

Preliminary Studies on the Food and Feeding Habits of Synodontis Membraneceus from Ogobiri River, Nigeria
r 233 Preliminary studies on the food and feeding habits of Synodontis membraneceus from Ogobiri River, Nigeria Allison, M. E. / Youdubagha, S. E. Abstract Thefood andfeeding habits of Synodontis membraneceus of Ogobiri River of Bayelsa State, Nigeria was studied using 44 male and 51 female specimens that were boughtfrom fishers in the study area measuring between 5 and 25 em total length. The Numerical and the Frequency of Occurrence method of analysis were used. For the males, thefood items by the numerical method were copepods (168), insects, (118), Cladocera (69), and unidentified items (32). In thefemale specimens, thefood items were Cladocera (286), Insect (216) Copepod (100) and unidentified organisms/materials (65). In the Frequency of Occurrence method for the males, Copepod was still the highest with a total of (48), Cladocera (44), Insects (29) and unidentified (I 6). For thefemales, Cladoera (48), Insects were (30) followed by Copepods (35) and unidentified (J9). The Percentage composition offood items by the Numerical method was Cladocera (45.5%), Insects (34.5%), Copepod (12.5%), and unidentified items (7.0%) while the Percentage Frequency of Occurrence was, Cla• docera (30.5%), Insects (30.9%), Copepod (29.6%), and unidentified items (9.0%). Keywords:Numerical method,frequency of occurrence, S. membraneceus, Ogobiri River. Introduction ish is a very important source of food for humans (FAO, 2010). Information about the food habits of fishes is on defin• ing predator-prey relationship (Saa et al., 1997) and the creation of trophic models as a tool to understanding complete Fecosystem (Lopez-Perata and Arcila, 2002; Bachok et al., 2004). -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Lates Niloticus) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Nile Perch (Lates niloticus) Ecological Risk Screening Summary Web Version – September 2014 Photo: © Biopix: N Sloth 1 Native Range, and Status in the United States Native Range From Schofield (2011): “Much of central, western and eastern Africa: Nile River (below Murchison Falls), as well as the Congo, Niger, Volga, Senegal rivers and lakes Chad and Turkana (Greenwood 1966 [cited by Schofield (2011) but not accessed for this report]). Also present in the brackish Lake Mariot near Alexandria, Egypt.” Lates niloticus Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version - 8/14/2012 Status in the United States From Schofield (2011): “Scientists from Texas traveled to Tanzania in 1974-1975 to investigate the introduction potential of Lates spp. into Texas reservoirs (Thompson et al. 1977 [cited by Schofield (2011) but not accessed for this report]). Temperature tolerance and trophic dynamics were studied for three species (L. angustifrons, L. microlepis and L. mariae). Subsequently, several individuals of these three species were shipped to Heart of the Hills Research Station (HOHRS) in Ingram, Texas in 1975 (Rutledge and Lyons 1976 [cited by Schofield (2011) but not accessed for this report]). Also in 1975, Nile perch (L. niloticus) were transferred from Lake Turkana, Kenya, to HOHRS. All fishes were held in indoor, closed-circulating systems (Rutledge and Lyons 1976).” “From 1978 to 1985, Lates spp. was released into various Texas reservoirs (Howells and Garrett 1992 [cited by Schofield (2011) but not accessed for this report]). Almost 70,000 Lates spp. larvae were stocked into Victor Braunig (Bexar Co.), Coleto Creek (Goliad Co.) and Fairfield (Freestone Co.) reservoirs between 1978 and 1984. -

Monophyly and Interrelationships of Snook and Barramundi (Centropomidae Sensu Greenwood) and five New Markers for fish Phylogenetics ⇑ Chenhong Li A, , Betancur-R
Molecular Phylogenetics and Evolution 60 (2011) 463–471 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Monophyly and interrelationships of Snook and Barramundi (Centropomidae sensu Greenwood) and five new markers for fish phylogenetics ⇑ Chenhong Li a, , Betancur-R. Ricardo b, Wm. Leo Smith c, Guillermo Ortí b a School of Biological Sciences, University of Nebraska, Lincoln, NE 68588-0118, USA b Department of Biological Sciences, The George Washington University, Washington, DC 200052, USA c The Field Museum, Department of Zoology, Fishes, 1400 South Lake Shore Drive, Chicago, IL 60605, USA article info abstract Article history: Centropomidae as defined by Greenwood (1976) is composed of three genera: Centropomus, Lates, and Received 24 January 2011 Psammoperca. But composition and monophyly of this family have been challenged in subsequent Revised 3 May 2011 morphological studies. In some classifications, Ambassis, Siniperca and Glaucosoma were added to the Accepted 5 May 2011 Centropomidae. In other studies, Lates + Psammoperca were excluded, restricting the family to Available online 12 May 2011 Centropomus. Recent analyses of DNA sequences did not solve the controversy, mainly due to limited taxonomic or character sampling. The present study is based on DNA sequence data from thirteen Keywords: genes (one mitochondrial and twelve nuclear markers) for 57 taxa, representative of all relevant Centropomidae species. Five of the nuclear markers are new for fish phylogenetic studies. The monophyly of Centrop- Lates Psammoperca omidae sensu Greenwood was supported by both maximum likelihood and Bayesian analyses of a Ambassidae concatenated data set (12,888 bp aligned). No support was found for previous morphological hypothe- Niphon spinosus ses suggesting that ambassids are closely allied to the Centropomidae. -

View/Download
CICHLIFORMES: Cichlidae (part 3) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 6.0 - 30 April 2021 Order CICHLIFORMES (part 3 of 8) Family CICHLIDAE Cichlids (part 3 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Haplochromis through Konia) Haplochromis Hilgendorf 1888 haplo-, simple, proposed as a subgenus of Chromis with unnotched teeth (i.e., flattened and obliquely truncated teeth of H. obliquidens); Chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), then beginning to be used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Haplochromis acidens Greenwood 1967 acies, sharp edge or point; dens, teeth, referring to its sharp, needle-like teeth Haplochromis adolphifrederici (Boulenger 1914) in honor explorer Adolf Friederich (1873-1969), Duke of Mecklenburg, leader of the Deutsche Zentral-Afrika Expedition (1907-1908), during which type was collected Haplochromis aelocephalus Greenwood 1959 aiolos, shifting, changing, variable; cephalus, head, referring to wide range of variation in head shape Haplochromis aeneocolor Greenwood 1973 aeneus, brazen, referring to “brassy appearance” or coloration of adult males, a possible double entendre (per Erwin Schraml) referring to both “dull bronze” color exhibited by some specimens and to what -

The Charcoal Grey Market in Kenya, Uganda and South Sudan (2021)
COMMODITY REPORT BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan SIMONE HAYSOM I MICHAEL McLAGGAN JULIUS KAKA I LUCY MODI I KEN OPALA MARCH 2021 BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan ww Simone Haysom I Michael McLaggan Julius Kaka I Lucy Modi I Ken Opala March 2021 ACKNOWLEDGEMENTS The authors would like to thank everyone who gave their time to be interviewed for this study. They would like to extend particular thanks to Dr Catherine Nabukalu, at the University of Pennsylvania, and Bryan Adkins, at UNEP, for playing an invaluable role in correcting our misperceptions and deepening our analysis. We would also like to thank Nhial Tiitmamer, at the Sudd Institute, for providing us with additional interviews and information from South Sudan at short notice. Finally, we thank Alex Goodwin for excel- lent editing. Interviews were conducted in South Sudan, Uganda and Kenya between February 2020 and November 2020. ABOUT THE AUTHORS Simone Haysom is a senior analyst at the Global Initiative Against Transnational Organized Crime (GI-TOC), with expertise in urban development, corruption and organized crime, and over a decade of experience conducting qualitative fieldwork in challenging environments. She is currently an associate of the Oceanic Humanities for the Global South research project based at the University of the Witwatersrand in Johannesburg. Ken Opala is the GI-TOC analyst for Kenya. He previously worked at Nation Media Group as deputy investigative editor and as editor-in-chief at the Nairobi Law Monthly. He has won several journalistic awards in his career. -

———— “Mudo”: the Soga 'Little Red Riding Hood'
LILLIAN BUKAAYI TIBASIIMA ———— º “Mudo”: The Soga ‘Little Red Riding Hood’ ABSTRACT This essay analyses the social underpinnings of the oral tale of “Mudo,” which belongs to the Aarne–Thompson tale type 333, along with a group of similar tales that resemble the action and movement of “Little Red Riding Hood.” Basic to the exposition is Adolf Bastian’s assertion of the fundamental similarity of ideas between all social groups. In the “Mudo” story and its Ugandan variants, the victim is a solitary little girl and the villain a male ogre who devises ways of eating her; the ogre is mostly successful, although in some variants the girl manages to escape. Although these tales come from a great range of cultures and different geographical locations, and the counterpart of the ogre in the European tales is a wolf in disguise, they share elements of plot, characteriza- tion, and motif, and address similar concerns. Introduction USOGA IS PART OF EAS TERN UGANDA, surrounded by water. The B Rev. Fredrick Kisuule Kaliisa1 notes: To the west is river Kiira (Nile) marking the boundary between Buganda and Busoga. To the East is river Mpologoma separating Busoga from Bukedi. To the North are river Mpologoma and Lake Kyoga, forming the boundary be- tween Busoga and Lango. To the south, is Lake Victoria (Nalubaale). It might be the result of the geographical location of Busoga that ogre stories were composed to warn the people against impending harm if they went out alone and stayed in secluded places. Nnalongo Lukude emphasizes this: Historically, Busoga was surrounded by bodies of water and forests, it was very bushy and as a result harboured many wild animals, some of which were man-eaters. -
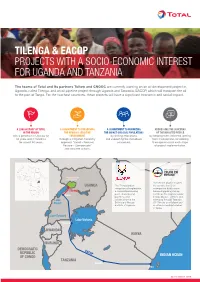
Tilenga & Eacop Projects with a Socio-Economic Interest for Uganda
TILENGA & EACOP PROJECTS WITH A SOCIO-ECONOMIC INTEREST FOR UGANDA AND TANZANIA The teams of Total and its partners Tullow and CNOOC are currently working on an oil development project in Uganda, called Tilenga, and an oil pipeline project through Uganda and Tanzania, EACOP, which will transport the oil to the port of Tanga. For the two host countries, these projects will have a significant economic and social impact. A LONG HISTORY OF TOTAL A COMMITMENT TO PRESERVING A COMMITMENT TO MINIMIZING ADDRESSING THE CONCERNS IN THE REGION THE REGION'S SENSITIVE THE IMPACT ON LOCAL POPULATIONS OF THE IMPACTED PEOPLE with a presence in Uganda for ENVIRONMENT by limiting relocations by keeping them informed, getting 60 years and in Tanzania through a mitigation hierarchy and supporting the individuals them involved and considering for almost 50 years. approach “Avoid – Reduce/ concerned. their opinions into each stage Restore – Compensate” of project implementation. and concrete actions. SOUDAN DU SUD ÉTHIOPIE Murchison Falls National Park The EACOP project involves UGANDA The Tilenga project the construction of an comprises oil exploration, underground hydrocarbon Tilenga a crude oil processing transport pipeline starting Hoima plant, underground just inside the Uganda border pipelines, and (Hoima District - 297km) and Lake infrastructure in the extending through Tanzania Albert Buliisa and Nwoya (1147km) to an oil depot and districts of Uganda. an offshore loading terminal in Tanga. Lake Edward Lake Victoria Bukoba RWANDA KENYA BURUNDI DEMOCRATIC EACOP REPUBLIC INDIAN OCEAN OF CONGO TANZANIA Singida Tanga SEPTEMBER 2019 ZAMBIE MOZAMBIQUE MALAWI SOUDAN DU SUD THIOPIE FOCUS ON THE TILENGA PROJECT Total E&P Uganda, fully aware of the project's sensitive nature, has placed particular emphasis on environmental and societal issues, with a specific commitment to leaving the site in a better state than it was before the work started and to limiting residents' relocations as much as possible. -

The Precolonial Social Formation Among the Bakenhe Fishing Community
The Precolonial Social Formation Among the Bakenhe Fishing Community .. of Lake Kyoga Il.egion of Uganda , 1800- 1894* • By C. Asowa- Okwe , Department of Political Science and Public Adminis t rat i on. Int roduction It i s eminently evident from the literqture ava ilable on the pre- colonia l history of Uganda and East Africa in general , that one area which has either been negl ected , or peripher ally treat ed i s that of the fishing industry and the fishing communities . A car eful and close examina tion of these liter a ture show a definite bia s towar ds the agricult ural and pastoral communities and their economic activities. And as if tha t is not enough , f ew that have t r i ed to grappl e with the fishing industry have l ar gely tended to be descriptive/narra tive , and ma inly, t alking a bout methods of fishing and the types of fis hes c aught. In f act the bulk of literature ~ on the fishing industry are basically , works of the physical scientists who ar e mainl y tra i ned in bi ol ogica l sciences . These studies , ther ef or e , ma i nly focus on the fish species f ound in the wa t ers of East African l akes , rivers , ponds and swamps , their f ood r equirements and dist ribut ion. They a lso concern thems elves with the question of density of fish popu- l a tion, the growth r a t e of i ndividua l species, the age at which they mature , t he specific f actor s which cause ornt~o p ic a l l ake to support many fish and another r el a tively f ew, the depletion of certa in fish species , the stocking of new species, and how t o check .the depl etion of s ome species, like·, ales.tes ( soga) , Labeo (ningu), bagrus (semutundu) . -

World Bank Document
KENYA Western Kenya Integrated Ecosystem Management Project Brief Africa Regional Office Public Disclosure Authorized AFTS2 Date: April 15 , 2004 Team Leader: Berhane Manna Sector Manager: Karen Mcconnell Brooks Country Manager/Director: Makhtar Diop Project ID: P072981 Focal Area: M - Multi-focal area Sector(s): General agriculture, fishing and forestry sector (100%) Theme(s): Environmental policies and institutions (P), Biodiversity (P), Other environment and natural resources Public Disclosure Authorized management (S) Project Financing Data [ ] Loan [ ] Credit [ X] Grant [ ] Guarantee [ x ] Other: For Loans/Credits/Others: Total Project Cost (US$m): $9.55 Cofinancing: 5.45 Total Bank Financing (US$m): 4.1 Proposed Terms (IDA): GEF Financing Plan (US$m): Source Local Foreign Total BORROWER 2.75 0.00 2.75 PHRD 0.40 0.00 0.40 SIDA 2.30 0.00 2.30 Public Disclosure Authorized GLOBAL ENVIRONMENT FACILITY 4.10 0.00 4.10 Total: 9.55 0.00 9.55 Borrower/Recipient: GOVERNMENT OF KENYA Responsible agency: KARI Kenya Agricultural Research Institute (KARI) Address: P.O. Box 57811 Nairobi, Kenya Contact Person: Dr. Romano Kiome, Director Tel: 254-2-583-290 Fax: 254 (0) 20583344 Email: [email protected] Project implementation period: 5 years Public Disclosure Authorized Table of Contents A. Project Development Objective .....................................................................................3 B. Strategic Context ............................................................................................................5 C. Project Description