Caring for Asian Small-Clawed, Cape Clawless, Nearctic, and Spotted-Necked Otters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seamscape a Procedural Generation System for Accelerated Creation of 3D Landscapes
SeamScape A procedural generation system for accelerated creation of 3D landscapes Bachelor Thesis in Computer Science and Engineering Link to video demonstration: youtu.be/K5yaTmksIOM Sebastian Ekman Anders Hansson Thomas Högberg Anna Nylander Alma Ottedag Joakim Thorén Chalmers University of Technology University of Gothenburg Department of Computer Science and Engineering Gothenburg, Sweden, June 2016 The Authors grants to Chalmers University of Technology and University of Gothenburg the non-exclusive right to publish the Work electronically and in a non-commercial purpose make it accessible on the Internet. The Author warrants that he/she is the author to the Work, and warrants that the Work does not contain text, pictures or other material that violates copyright law. The Author shall, when transferring the rights of the Work to a third party (for example a publisher or a company), acknowledge the third party about this agreement. If the Author has signed a copyright agreement with a third party regarding the Work, the Author warrants hereby that he/she has obtained any necessary permission from this third party to let Chalmers University of Technology and University of Gothenburg store the Work electronically and make it accessible on the Internet. SeamScape A procedural generation system for accelerated creation of 3D landscapes Sebastian Ekman Anders Hansson Thomas Högberg Anna Nylander Alma Ottedag Joakim Thorén c Sebastian Ekman, 2016. c Anders Hansson, 2016. c Thomas Högberg, 2016. c Anna Nylander, 2016. c Alma Ottedag, 2016. -
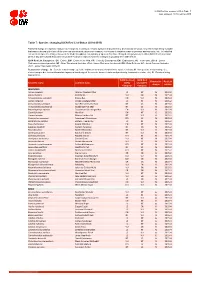
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Coping with the Bounds: a Neo-Clausewitzean Primer / Thomas J
About the CCRP The Command and Control Research Program (CCRP) has the mission of improving DoD’s understanding of the national security implications of the Information Age. Focusing upon improving both the state of the art and the state of the practice of command and control, the CCRP helps DoD take full advantage of the opportunities afforded by emerging technologies. The CCRP pursues a broad program of research and analysis in information superiority, information operations, command and control theory, and associated operational concepts that enable us to leverage shared awareness to improve the effectiveness and efficiency of assigned missions. An important aspect of the CCRP program is its ability to serve as a bridge between the operational, technical, analytical, and educational communities. The CCRP provides leadership for the command and control research community by: • articulating critical research issues; • working to strengthen command and control research infrastructure; • sponsoring a series of workshops and symposia; • serving as a clearing house for command and control related research funding; and • disseminating outreach initiatives that include the CCRP Publication Series. This is a continuation in the series of publications produced by the Center for Advanced Concepts and Technology (ACT), which was created as a “skunk works” with funding provided by the CCRP under the auspices of the Assistant Secretary of Defense (NII). This program has demonstrated the importance of having a research program focused on the national security implications of the Information Age. It develops the theoretical foundations to provide DoD with information superiority and highlights the importance of active outreach and dissemination initiatives designed to acquaint senior military personnel and civilians with these emerging issues. -

Rio Grande National Forest Draft Assessment 5 At-Risk Species
Rio Grande National Forest- Draft Assessment 5 Identifying and Assessing At-risk Species Rio Grande National Forest Draft Assessment 5 Identifying and Assessing At-risk Species Contents Introduction .............................................................................................................................................. 1 Information Sources and Gaps .............................................................................................................. 2 Existing Forest Plan Direction .............................................................................................................. 2 Scale of Analysis (Area of Influence) ................................................................................................... 4 Assessment 5 Development Process ..................................................................................................... 4 Federally Recognized Species .................................................................................................................. 6 Uncompahgre Fritillary Butterfly ......................................................................................................... 6 Black-footed Ferret ............................................................................................................................... 8 Canada Lynx ....................................................................................................................................... 11 New Mexico Meadow Jumping Mouse ............................................................................................. -

VOLATILE COMPOUNDS from ANAL GLANDS of the WOLVERINE, Gulo Gulo
Journal of Chemical Ecology, Vol. 12, No. 9, September 2005 ( #2005) DOI: 10.1007/s10886-005-6080-9 VOLATILE COMPOUNDS FROM ANAL GLANDS OF THE WOLVERINE, Gulo gulo WILLIAM F. WOOD,1,* MIRANDA N. TERWILLIGER,2 and JEFFREY P. COPELAND3 1Department of Chemistry, Humboldt State University, Arcata, CA 95521, USA 2Alaska Cooperative Fish & Wildlife Research Unit, Department of Biology & Wildlife, University of Alaska, Fairbanks, AK 99775, USA 3USDA Forest Service, Rocky Mountain Research Station, Missoula, MT 59801, USA (Received February 12, 2005; revised March 24, 2005; accepted April 20, 2005) Abstract—Dichloromethane extracts of wolverine (Gulo gulo, Mustelinae, Mustelidae) anal gland secretion were examined by gas chromatographyYmass spectrometry. The secretion composition was complex and variable for the six samples examined: 123 compounds were detected in total, with the number per animal ranging from 45 to 71 compounds. Only six compounds were common to all extracts: 3-methylbutanoic acid, 2-methylbutanoic acid, phenylacetic acid, a-tocopherol, cholesterol, and a compound tentatively identified as 2-methyldecanoic acid. The highly odoriferous thietanes and dithiolanes found in anal gland secretions of some members of the Mustelinae [ferrets, mink, stoats, and weasels (Mustela spp.) and zorillas (Ictonyx spp.)] were not observed. The composition of the wolverine’s anal gland secretion is similar to that of two other members of the Mustelinae, the pine and beech marten (Martes spp.). Key WordsVWolverine, Gulo gulo, Mustelinae, Mustelidae, scent marking, fear-defense mechanism, short-chain carboxylic acids. INTRODUCTION The wolverine (Gulo gulo) is the largest terrestrial member of the Mustelidae and is part of the subfamily, Mustelinae, which includes ferrets, fishers, martens, mink, stoats, weasels, and zorillas. -

Jon Fauer ASC Issue 109
Jon Fauer ASC www.fdtimes.com Aug 2021 Issue 109 Art, Technique and Technology in Motion Picture Production Worldwide Aug 2021 • Issue 109 1 www.fdtimes.com Art, Technique and Technology On Paper, Online, and now on iPad Film and Digital Times is the guide to technique and technology, tools and how-tos for Cinematographers, Photographers, Directors, Producers, Studio Executives, Camera Assistants, Camera Operators, Grips, Gaffers, Crews, Rental Houses, and Manufacturers. Subscribe It’s written, edited, and published by Jon Fauer, ASC, an award-winning Cinematographer and Director. He is the author of 14 bestselling Online: books—over 120,000 in print—famous for their user-friendly way of explaining things. With inside-the-industry “secrets-of the-pros” www.fdtimes.com/subscribe information, Film and Digital Times is delivered to you by subscription or invitation, online or on paper. We don’t take ads and are supported by readers and sponsors. Call, Mail or Fax: © 2021 Film and Digital Times, Inc. by Jon Fauer Direct Phone: 1-570-567-1224 Toll-Free (USA): 1-800-796-7431 subscribe Fax: 1-724-510-0172 Film and Digital Times Subscriptions www.fdtimes.com PO Box 922 Subscribe online, call, mail or fax: Williamsport, PA 17703 Direct Phone: 1-570-567-1224 USA Toll-Free (USA): 1-800-796-7431 1 Year Print and Digital, USA 6 issues $ 49.95 1 Year Print and Digital, Canada 6 issues $ 59.95 Fax: 1-724-510-0172 1 Year Print and Digital, Worldwide 6 issues $ 69.95 1 Year Digital (PDF) $ 29.95 1 year iPad/iPhone App upgrade + $ 9.99 Film and Digital Times (normally 29.99) Get FDTimes on Apple Newsstand with iPad App when you order On Paper, Online, and On iPad a Print or Digital Subscription (above) Total $ __________ Print + Digital Subscriptions Film and Digital Times Print + Digital subscriptions continue to Payment Method (please check one): include digital (PDF) access to current and all back issues online. -

Demography of the Giant Otter (Pteronura Brasiliensis) in Manu National Park, South-Eastern Peru: Implications for Conservation
Demography of the Giant Otter (Pteronura brasiliensis) in Manu National Park, South-Eastern Peru: Implications for Conservation Jessica Groenendijk1,3,5*, Frank Hajek2,5, Paul J. Johnson3, David W. Macdonald3, Jorge Calvimontes4,5, Elke Staib5, Christof Schenck5 1 San Diego Zoo Global Peru, Department of Cusco, Cusco, Peru´, 2 Nature Services Peru, Department of Cusco, Cusco, Peru´, 3 Wildlife Conservation Research Unit, University of Oxford, Abingdon, Oxfordshire, United Kingdom, 4 Environmental Studies and Research Center, University of Campinas, Sa˜o Paulo, Brazil, 5 Frankfurt Zoological Society, Frankfurt, Germany Abstract The giant otter (Pteronura brasiliensis) is an endangered semi-aquatic carnivore of South America. We present findings on the demography of a population inhabiting the floodplain of Manu National Park, south-eastern Peru, arising from 14 annual dry season censuses over a 16 year period. The breeding system of territorial groups, including only a single breeding female with non-reproductive adult ‘helpers’, resulted in a low intrinsic rate of increase (0.03) and a slow recovery from decades of hunting for the pelt trade. This is explained by a combination of factors: (1) physiological traits such as late age at first reproduction and long generation time, (2) a high degree of reproductive skew, (3) small litters produced only once a year, and (4) a 50% mortality between den emergence and age of dispersal, as well as high mortality amongst dispersers (especially males). Female and male giant otters show similar traits with respect to average reproductive life- spans (female 5.4 yrs., male 5.2 yrs.) and average cub productivity (female 6.9, male 6.7 cubs per lifetime); the longest reproductive life spans were 11 and 13 years respectively. -

African Clawless Otter (Aonyx Capensis) – Near Threatened (IUCN Red List)
African clawless otter (Aonyx capensis) – Near Threatened (IUCN Red List) The Cape clawless otter is large: head and body about 1 m long, tail 50 cm, and typically weighs 10 - 20 kg. Upper parts of the body are dark brown, and the chin, cheeks, throat, and chest off- white. Whiskers are very long, numerous, and white. They assist in detecting its main prey (crabs) which are captured with the dexterous clawless forefeet. In freshwater habitats, it feeds mostly on crabs, and lesser amounts of frogs, fish, and seasonally on dragonfly larvae. Otters dry and groom themselves by rolling in grass, or on sandbanks or rocks. At most rolling places latrines are established, which like the rolling places, are regularly used. While defecating a clawless otter often rotates on the spot, so the scats are dropped all around it, broken into short pieces, not tapered at both ends. When fresh scats are dark brown, changing to a cream colour as they dry out; typically, about 25 mm diameter, range 22 - 30 mm. Track is 60 – 90mm wide. Male’s larger than female’s African Otter Network – africanotternetwork.wordpress.com – African clawless Fresh scat – 20 - 32mm diameter, typically full of crab, maybe some fish. No seeds, hair. Otter scat – fresh (left); aged (below) Otter scat can be confused with marsh mongoose scat (right). Mongoose scat is smaller 15 – 22mm diameter and consists of crab, small mammal (hair), insect, bird feathers Marsh mongoose scat The African Otter Network is looking for information on current distribution: if seen please report to: [email protected] Where, when, how many, and any other observation information. -

Otter News No. 124, July 2021
www.otter.org IOSF Otter News No. 124, July 2021 www.loveotters.org Otter News No. 124, July 2021 Join our IOSF mailing list and receive our newsletters - Click on this link: http://tinyurl.com/p3lrsmx Please share our news Good News for Otters in Argentina Giant otters are classified as “extinct” in Argentina but there have been some positive signs of their return in recent months. The Ibera wetlands lie in the Corrientes region and are one of the world’s largest freshwater ecosystems. Rewilding Argentina is attempting to return the country’s rich biodiversity to the area with species such as jaguars, macaws and marsh deer. They have also been working to bring back giant otters and there have been some small successes and three cubs have recently been born as offspring of two otters that were reintroduced there. And there is more good news for the largest otter species. In May there was the first sighting of “wild” giant otters in Argentina for 40 years! Furthermore, there have been other success stories for otters across the south American nation. Tierra del Fuego, Argentina’s southern-most province, has banned all open-net salmon farming. This ban will help protect the areas fragile marine ecosystems, which is home to half of Argentina’s kelp forests which support species such as the southern river otter. This also makes Argentina the first nation in the world to ban such farming practices. With so many problems for otter species it is encouraging to see some steps forward in their protection in Argentina. -

Monitoring Wolverines in Northeast Oregon
Monitoring Wolverines in Northeast Oregon January 2011 – December 2012 Final Report Authors: Audrey J. Magoun Patrick Valkenburg Clinton D. Long Judy K. Long Submitted to: The Wolverine Foundation, Inc. February 2013 Cite as: A. J. Magoun, P. Valkenburg, C. D. Long, and J. K. Long. 2013. Monitoring wolverines in northeast Oregon. January 2011 – December 2012. Final Report. The Wolverine Foundation, Inc., Kuna, Idaho. [http://wolverinefoundation.org/] Copies of this report are available from: The Wolverine Foundation, Inc. [http://wolverinefoundation.org/] Oregon Department of Fish and Wildlife [http://www.dfw.state.or.us/conservationstrategy/publications.asp] Oregon Wildlife Heritage Foundation [http://www.owhf.org/] U. S. Forest Service [http://www.fs.usda.gov/land/wallowa-whitman/landmanagement] Major Funding and Logistical Support The Wolverine Foundation, Inc. Oregon Department of Fish and Wildlife Oregon Wildlife Heritage Foundation U. S. Forest Service U. S. Fish and Wildlife Service Wolverine Discovery Center Norcross Wildlife Foundation Seattle Foundation Wildlife Conservation Society National Park Service 2 Special thanks to everyone who provided contributions, assistance, and observations of wolverines in the Wallowa-Whitman National Forest and other areas in Oregon. We appreciate all the help and interest of the staffs of the Oregon Department of Fish and Wildlife, Oregon Wildlife Heritage Foundation, U. S. Forest Service, U. S. Fish and Wildlife Service, Wildlife Conservation Society, and the National Park Service. We also thank the following individuals for their assistance with the field work: Jim Akenson, Holly Akenson, Malin Aronsson, Norma Biggar, Ken Bronec, Steve Bronson, Roblyn Brown, Vic Coggins, Alex Coutant, Cliff Crego, Leonard Erickson, Bjorn Hansen, Mike Hansen, Hans Hayden, Tim Hiller, Janet Hohmann, Pat Matthews, David McCullough, Glenn McDonald, Jamie McFadden, Kendrick Moholt, Mark Penninger, Jens Persson, Lynne Price, Brian Ratliff, Jamie Ratliff, John Stephenson, John Wyanens, Rebecca Watters, Russ Westlake, and Jeff Yanke. -

Care of the Pet Ferret
Care of the Pet Ferret What is a ferret? Ferrets are playful, friendly animals that can make excellent pets for the right person. • A ferret may not be the best pet for a family with young children. Interactions between ferrets (or any pet!) and a young child should always be monitored. • Ferrets also tend to get along well with most cats and dogs, however this predator species may not get along with birds, rabbits, rodents, or lizards. • Some states, counties, and cities carry restrictions on the ownership of ferrets or require permits. Be sure to research the law in your area! The ferret is a relative of the weasel, skunk, and otter. Most ferrets sold as pets in the United States come from a commercial breeding farm where young ferrets or “kits” are neutered and their anal musk glands are removed (descented). Two small blue tattoos are placed in the ear at the same time these procedures are performed. Although pet ferrets are descented, they still retain their natural musky odor. Ferrets live an average 6 to 8 years. Females typically weigh between 500 and 900 grams (1.1-2 lb) while males generally weigh 800 to 1200 grams (1.7-2.6 lb). Feeding your ferret The ferret is a strict carnivore that requires a diet rich in animal protein (30% to 40%) and fat (15% to 20%) plus approximately 2% fiber. Most ferret foods or a high-quality dry cat food (e.g. Science Diet, Iams) meet their nutritional requirements. Most ferrets eat many small meals in a day, so make food available at all times. -

Clinical Practice Guideline for the Management of Anorectal Abscess, Fistula-In-Ano, and Rectovaginal Fistula Jon D
PRACTICE GUIDELINES Clinical Practice Guideline for the Management of Anorectal Abscess, Fistula-in-Ano, and Rectovaginal Fistula Jon D. Vogel, M.D. • Eric K. Johnson, M.D. • Arden M. Morris, M.D. • Ian M. Paquette, M.D. Theodore J. Saclarides, M.D. • Daniel L. Feingold, M.D. • Scott R. Steele, M.D. Prepared on behalf of The Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons he American Society of Colon and Rectal Sur- and submucosal locations.7–11 Anorectal abscess occurs geons is dedicated to ensuring high-quality pa- more often in males than females, and may occur at any Ttient care by advancing the science, prevention, age, with peak incidence among 20 to 40 year olds.4,8–12 and management of disorders and diseases of the co- In general, the abscess is treated with prompt incision lon, rectum, and anus. The Clinical Practice Guide- and drainage.4,6,10,13 lines Committee is charged with leading international Fistula-in-ano is a tract that connects the perine- efforts in defining quality care for conditions related al skin to the anal canal. In patients with an anorec- to the colon, rectum, and anus by developing clinical tal abscess, 30% to 70% present with a concomitant practice guidelines based on the best available evidence. fistula-in-ano, and, in those who do not, one-third will These guidelines are inclusive, not prescriptive, and are be diagnosed with a fistula in the months to years after intended for the use of all practitioners, health care abscess drainage.2,5,8–10,13–16 Although a perianal abscess workers, and patients who desire information about the is defined by the anatomic space in which it forms, a management of the conditions addressed by the topics fistula-in-ano is classified in terms of its relationship to covered in these guidelines.