| Hai Lala Mo at an Uit Dituri a to Od
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CREATOR 100 Per Box
UNISEX T-SHIRT STTU755 - CREATOR 100 per box Iconic Unisex T-Shirt Singlejersey 100% gekämmte ringgesponnene Bio-Baumwolle Normale Passform 180 GSM . Eingesetzte Ärmel . 1x1 Rippstrick am Halsausschnitt . Abgesetztes Nackenband . Breite Doppelabsteppung an Ärmelenden und unterem Saum C WHITES 2.5cm below A armhole B WHITE OFF WHITE VINTAGE WHITE NATURAL RAW COLORS FRONT BACK SIZES XXS XS S M L XL XXL XXXL XXXXL XXXXXL BLACK ANTHRACITE OPAL DESERT DUST CAMEL DEEP CHOCOLATE CARAMEL MUSHROOM OCHRE A Half Chest 43.5 46 49 52 55 58 61 64 69 74 B Body Length 64 66 69 72 74 76 78 80 82 84 C Sleeve Length 19 19.5 20.5 21.5 22.5 22.5 23.5 24.5 24.5 25 Some sizes are only available on a limited number of colours. Please check our webshop for latest information SPECTRA YELLOW GOLDEN YELLOW FRESH GREEN VARSITY GREEN GLAZED GREEN BOTTLE GREEN BRITISH KHAKI KHAKI SAGE Combo options STEM GREEN CARIBBEAN BLUE TEAL MONSTERA OCEAN DEPTH STARGAZER CITADEL BLUE BABY BLUE SERENE BLUE SKY BLUE BRIGHT BLUE AZUR ROYAL BLUE MAJORELLE BLUE INDIA INK GREY FRENCH NAVY PINK PUNCH CARMINE RED RED Mini Creator Stella Expresser BURGUNDY PLUM LAVENDER DAWN LILAC PETAL MAUVE CANDY PINK COTTON PINK ROSE CLAY MELON CODE Printing Specifications NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW Eignet sich für alle Drucktechniken. Mehr Informationen finden Sie auf unserer Website. BRIGHT ORANGE TANGERINE BRIGHT RED JOJOBA INDIGO HUSH LAVENDER ORCHID FLOWER DUSTY MINT HIBISCUS ROSE Certifications & memberships ESSENTIAL HEATHERS DARK HEATHER MID HEATHER GREY HEATHER GREY CREAM HEATHER HEATHER SAND MID HEATHER MID HEATHER HEATHER ICE BLUE MID HEATHER BLUE GREY GREY KHAKI GREEN Wash Instructions Mit ähnlichen Farben waschen, Aufdruck nicht bügeln, auf links waschen und bügeln. -

CREATOR 100 Per Box
UNISEX T-SHIRTS CREATOR 100 per box STTU755 The iconic unisex t-shirt Single Jersey 100% Organic ring-spun combed cotton 180 GSM . Set-in sleeve . 1x1 rib at neck collar . Inside back neck tape in self fabric . Sleeve hem and bottom hem with wide double needle topstitch MEDIUM FIT WHITES XXS* XS S M L XL XXL XXXL* XXXXL* XXXXXL* NEWNEW Size guide NATURAL RAW VINTAGE WHITE OFF WHITE WHITE COLORS C NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW 2.5cm below A armhole CARAMEL BRIGHT BLUE MELON CODE CARMINE RED SAGE ROSE CLAY OCHRE MAUVE BRIGHT RED NEWNEW B FRONT BACK TANGERINE BRIGHT ORANGE SUNSET ORANGE CANDY PINK BURGUNDY RED PINK PUNCH COTTON PINK LAVENDER DAWN SIZES XXS XS S M L XL XXL XXXL XXXXL XXXXXL A Half Chest 43.5 46 49 52 55 58 61 64 69 74 PLUM MAJORELLE BLUE ROYAL BLUE FRENCH NAVY AZUR SKY BLUE BABY BLUE CITADEL BLUE STARGAZER B Body Length 64 66 69 72 74 76 78 80 82 84 C Sleeve Length 19 19.5 20.5 21.5 22.5 22.5 23.5 24.5 24.5 25 OCEAN DEPTH CARIBBEAN BLUE FRESH GREEN VARSITY GREEN GOLDEN YELLOW SPECTRA YELLOW HAY YELLOW MOSS GREEN BRITISH KHAKI Combo options KHAKI GLAZED GREEN BOTTLE GREEN DEEP CHOCOLATE ROASTED ORANGE CAMEL DESERT DUST OPAL ANTHRACITE INDIA INK GREY BLACK Stella Expresser Mini Creator ESSENTIAL HEATHERS Printing Specifications Fits all kinds of print techniques. Please download our printing guide for CREAM HEATHER MID HEATHER RED DARK HEATHER DARK HEATHER MID HEATHER BLUE HEATHER ICE BLUE MID HEATHER MID HEATHER HEATHER SAND PINK INDIGO BLUE GREEN KHAKI details. -

—Hence, Tirade
e. —hence , t irad to: literary a misfits digital monthly from: robocup zine press FEBRUARY 2018 | THE LOVE + HATE ISSUE. I don't know how to write love letters. -Frida Kahlo t w o table of contents plasmatic valentine 2 by tamryn spruill micro poem from The Twitter Hive Mind Is Dreaming 5 by jackie wang lost color 6 by katy ilonka i do 7 by judy swann love & hate playlists 8 by nottheboyevan monthly [horoscapes] 10 by matthew burnside blue is the warmest color movie review 12 by her head in films knock knock 13 by j. bradley coming next month ... 14 tuesday 15 by adrian slonaker nothing more to tell 16 by mitchell krockmalnik grabois the collection 20 by sarah etlinger contributor bios 22 acknowledgments 25 —hence, tirade. f SUBMIT o SUBSCRIBE u r www.robocup-press.com Copyright 2018 © Robocup Press. I'm beingI'm being cooked from thecooked from the inside by myinside by my desire. desire. -Jackie Wang Pre-orders for her forthcoming Twitter-poem collection begin 3/3! 5 Lost Color Katy Ilonka I cannot find it. Pushed bark into bloodshot birds and the irises that smell of the times you were here and it has gone. I have heard of a professor who studies the passage of time building a machine that brings the eyes into a single space and places the color precisely. I have heard of a yogi mechanic who adjusts the color with a chakra wrench until it glows just right. I have heard of the times our steps were in sync. -

Home Collection Fall Winter 2021
HOME COLLECTION FALL WINTER 2021 INDEX 02 HERITAGE COLLECTION TABLEWARE RED 70 TABLEWARE 07 TABLEWARE GIFT SETS 72 GLASSWARE 73 10 WINTER WONDERLAND KITCHEN- & TABLE TEXTILES 75 COLLECTION TABLEWARE 16 76 HOME DECORATIONS TABLEWARE GIFT SETS 18 COLLECTION GLASSWARE 19 CANDLE HOLDERS GLASS 76 OVEN- & BAKEWARE 21 CUSHIONS 86 KITCHEN- & TABLE TEXTILES 22 90 ROYAL COLLECTION CHRISTMAS DECORATIONS 25 ROYAL YERSEKE COLLECTION TABLEWARE 95 28 LOVEBIRDS COLLECTION ROYAL STRIPES COLLECTION TABLEWARE 96 TABLEWARE WHITE 32 ROYAL WHITE COLLECTION TABLEWARE 102 TABLEWARE BLUE-KHAKI 34 GLASSWARE 104 TABLEWARE RED-PINK 36 TABLEWARE GIFT SETS 105 TABLEWARE GIFT SETS WHITE 38 ROYAL COLOUR COLLECTION TABLEWARE GIFT SETS RED-PINK 40 TABLEWARE 108 TABLEWARE GIFT SETS BLUE-KHAKI 41 TABLEWARE GIFT SETS 111 HOME DECO METAL 112 42 JOLIE COLLECTION TABLEWARE 46 114 EARLY BIRD COLLECTION TABLEWARE GIFT SETS 48 TABLEWARE BLUE 116 GLASSWARE 49 TABLEWARE KHAKI 117 TABLEWARE PINK 118 50 LA MAJORELLE TABLEWARE GIFT SETS 119 COLLECTION GLASSWARE 119 TABLEWARE PINK 54 TABLEWARE BLUE 56 121 ALPHABET MUGS TABLEWARE GIFT SETS 58 122 BAGS COLLECTION GLASSWARE 58 COSMETIC BAGS 125 60 BLUSHING BIRDS BATHROOM ACCESSOIRES 126 COLLECTION FASHION- & TRAVEL BAGS 130 TABLEWARE BLUE 64 PROMO BAGS 136 TABLEWARE WHITE 66 SUSTAINABILITY, CARE & USE 137 TABLEWARE KHAKI 68 COLOPHON 138 HERITAGE COLLECTION Since 2007, Pip takes her fans on an incredible journey, always looking for new stories & inspirations. The unique designs for everyday products are typical Pip, and clearly recognizable with eye for detail, special finishes and true to the intuition of Anke, the founding lady. The stories are the starting point and the soul of the brand, always inspired on real life experiences! Every product is guaranteed to bring a sparkle to everyday life. -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin -

Colour Card Whites Colours
COLOUR CARD WHITES COLOURS As colour specialists, we strive every single day to achieve the right tone for the right garment. From rich, earthy tones to light, airy neutrals and pops of bright, vivid colour, we offer an ever-expanding range of classic and on-trend colours. FLUO FLUO Our colour range is divided into: WHITES NEW / SEASONAL SS20* NEW / SEASONAL SS20* NEW / SEASONAL SS20* NEW COLOURS C007 C504 C018 C011 C032 C013 C044 C042 C809 C038 ESSENTIAL HEATHERS Natural Vintage White Off White Bright Red Tangerine Bright Orange G Dyed Fluo Juicy Melon G Dyed Fluo Pink Crush Tie&Dye Canyon Pink Canyon Pink SPECIAL HEATHERS ALL OVER PRINTS For specific details on colour profiles (Pantone, CMYK and RGB references) make sure to consult the Colour Guide on NEW api.stanleystella.com C507 C001 C512 C016 C005 C024 C240 C004 C244 G Dyed White White G Dyed Canyon Pink Candy Pink Cotton Pink Pink Punch Raspberry Red Burgundy NEW / SEASONAL SS20* NEW NEW C241 C030 C810 C231 C230 C511 C034 Plum Lavender Dawn Tie&Dye Majorelle Blue Azur Royal Blue G Dyed Cadet Blue Majorelle Blue C003 C727 C551 C591 C552 C572 C571 Navy French Navy Mid Washed Indigo Midnight Blue Dark Washed Indigo Dark Indigo Denim Mid Indigo Denim *Seasonal colour - available until mid-2020, or while stocks last 243 244 COLOURS COLOURS NEW NEW C570 C232 C015 C725 C702 C710 C724 C514 C039 C715 C002 Light Indigo Denim Sky blue Baby Blue Citadel Blue Stargazer Ocean Depth Caribbean Blue G Dyed Lava Grey Lava Grey India Ink Grey Black FLUO FLUO NEW / SEASONAL SS20* NEW / SEASONAL SS20* -

Swatch Name HLS RGB HEX Absolute Zero 217° 36% 100% 0 72
Swatch Name HLS RGB HEX Absolute Zero 217° 36% 100% 0 72 186 #0048BA Acid green 65° 43% 76% 176 191 26 #B0BF1A Aero 206° 70% 70% 124 185 232 #7CB9E8 Aero blue 151° 89% 100% 201 255 229 #C9FFE5 African violet 288° 63% 31% 178 132 190 #B284BE Air superiority blue 205° 60% 39% 114 160 193 #72A0C1 Alabaster 46° 90% 27% 237 234 224 #EDEAE0 Alice blue 208° 97% 100% 240 248 255 #F0F8FF Alloy orange 27° 42% 85% 196 98 16 #C46210 Almond 30° 87% 52% 239 222 205 #EFDECD Amaranth 348° 53% 78% 229 43 80 #E52B50 Amaranth (M&P) 328° 40% 57% 159 43 104 #9F2B68 Amaranth pink 338° 78% 75% 241 156 187 #F19CBB Amaranth purple 342° 41% 63% 171 39 79 #AB274F Amaranth red 356° 48% 73% 211 33 45 #D3212D Amazon 147° 35% 35% 59 122 87 #3B7A57 Amber 45° 50% 100% 255 191 0 #FFBF00 Amber (SAE/ECE) 30° 50% 100% 255 126 0 #FF7E00 Amethyst 270° 60% 50% 153 102 204 #9966CC Android green 74° 50% 55% 164 198 57 #A4C639 Antique brass 22° 63% 47% 205 149 117 #CD9575 Antique bronze 52° 26% 55% 102 93 30 #665D1E Antique fuchsia 316° 46% 22% 145 92 131 #915C83 Antique ruby 350° 31% 66% 132 27 45 #841B2D Antique white 34° 91% 78% 250 235 215 #FAEBD7 Ao (English) 120° 25% 100% 0 128 0 #008000 Apple green 74° 36% 100% 141 182 0 #8DB600 Apricot 24° 84% 90% 251 206 177 #FBCEB1 Aqua 180° 50% 100% 0 255 255 #00FFFF Aquamarine 160° 75% 100% 127 255 212 #7FFFD4 Swatch Name HLS RGB HEX Arctic lime 72° 54% 100% 208 255 20 #D0FF14 Army green 69° 23% 44% 75 83 32 #4B5320 Artichoke 76° 53% 13% 143 151 121 #8F9779 Arylide yellow 51° 67% 74% 233 214 107 #E9D66B Ash gray 135° 72% 8% 178 190 -

Colour Guide 2020 Index
COLOUR GUIDE 2020 INDEX COLOURS 1 - New colours AW20 Go to 2 - All colours Go to In this colour guide you will find the Pantone, CMYK and RGB Colour code references for the Stanley/Stella colour range. Stanley/Stella products are priced according to colour group: IMPORTANT Colour equivalences between TCX and C are provided by my 1 - Whites Pantone™ X-ref. CMYK and RGB references have been defined 2 - Colours to match our fabric colours as accurately as possible. 3 - Essential Heathers 4 - Special Heathers Certain colours (heathers, fluos, tie & dye and garment dyed 5 - All over prints (AOP) colours) are difficult to translate into accurate colour references. Colour Name - C000 - 1 If you have any doubts in relation to a colour we recommend that C 00 Pantone R 000 you order a sample. M 00 00-0000 TCX G 000 Y 00 0000 C B 00 Colour appearance may vary depending on the screen. K 00 2 NEW COLOURS - AW20 Melon Code - C051 - 2 G-Dyed Carmin Red - C517 - 2 C 00 C 07 Pantone R 237 Pantone R 192 M 61 M 70 16-1453 TCX G 136 18-1629 TCX G 101 Y 72 Y 50 2026 C B 77 4061 C B 101 K 00 K 05 Rose Clay- C050 - 2 Mauve - C049 - 2 C 08 C 26 Pantone R 201 Pantone R 153 M 53 M 62 16-1431 TCX G 138 18-1710 TCX G 104 Y 47 Y 23 4052 C B 121 2055 C B 125 K 05 K 19 G-Dyed Aged Rose Clay - C522 - 2 G-Dyed Aged Mauve - C523 - 2 C 04 C 21 Pantone R 216 Pantone R 168 M 44 M 52 16-1330 TCX G 161 17-1512 TCX G 126 Y 39 Y 25 7606 C B 144 5005 C B 140 K 01 K 15 Heather Neppy Pink - C688 - 4 Tie&Dye Mauve/Rose Clay - C811 - 5 C 07 C 26 Pantone R 227 Pantone R 153 M 27 M -

(12) Patent Application Publication (10) Pub. No.: US 2014/0350127 A1 CANO Et Al
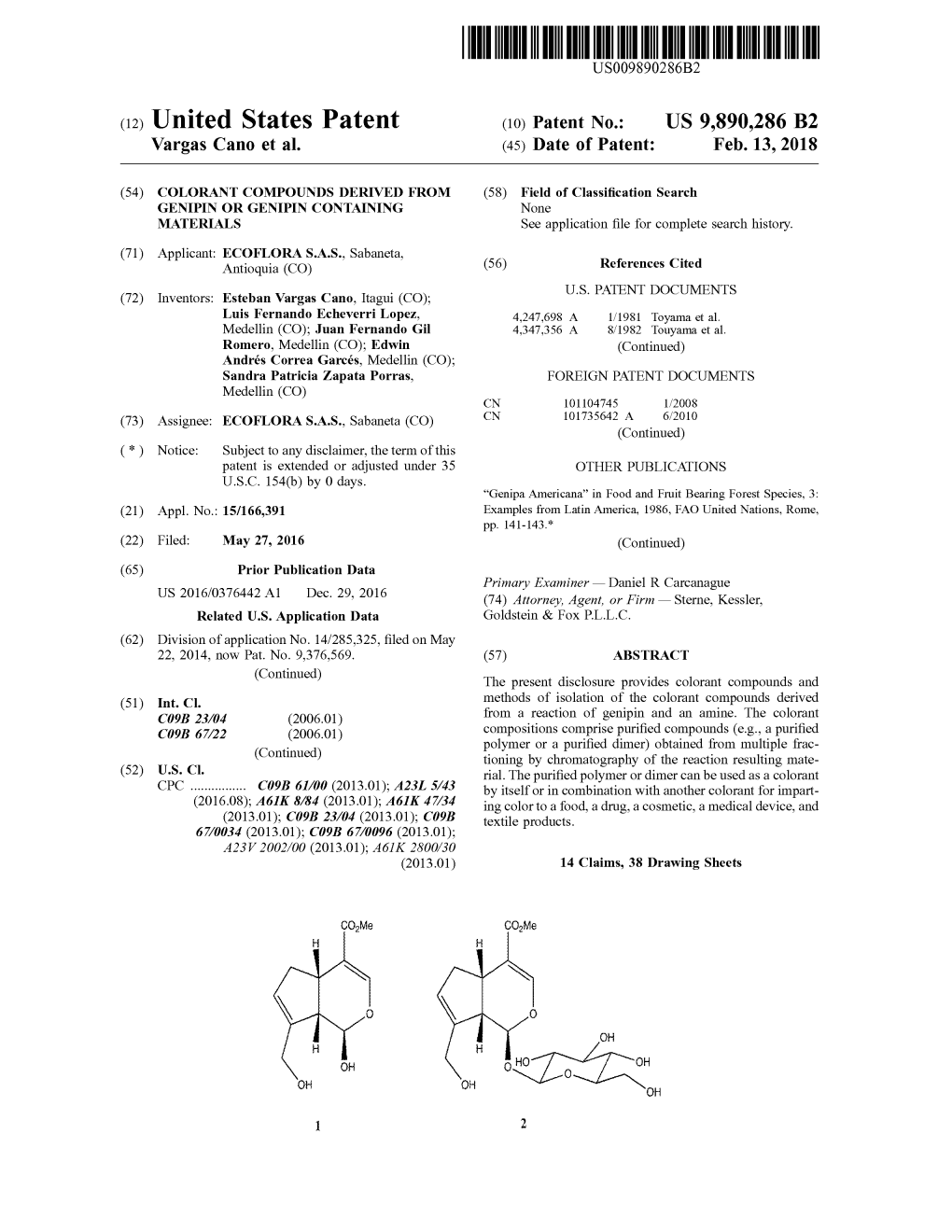
US 2014035O127A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0350127 A1 CANO et al. (43) Pub. Date: Nov. 27, 2014 (54) COLORANT COMPOUNDS DERVED FROM Publication Classification GENPIN OR GENPIN CONTAINING MATERLALS (51) Int. Cl. C09B 23/04 (2006.01) (71) Applicant: ECOFLORA S.A.S., Sabaneta (CO) C09B 67/54 (2006.01) C09B 67/22 (2006.01) (72) Inventors: Esteban Vargas CANO, Itagui (CO); (52) U.S. Cl. Luis Fernando Echeverri Lopez, CPC ............. C09B 23/04 (2013.01); C09B 67/0034 Medellin (CO); Juan Fernando Gil (2013.01); C09B 67/0096 (2013.01) Romero, Medellin (CO); Edwin Andrés USPC ........ 514/772.7:528/327: 528/321:524/879; Correa Garcés, Medellin (CO); Sandra 435/267; 426/540 Patricia Zapata Porras, Medellin (CO) (57) ABSTRACT (73) Assignee: ECOFLORA S.A.S., Sabaneta (CO) The present disclosure provides colorant compounds and methods of isolation of the colorant compounds derived from (21) Appl. No.: 14/285,325 a reaction of genipin and an amine. The colorant composi tions comprise purified compounds (e.g., a purified polymer (22) Filed: May 22, 2014 or a purified dimer) obtained from multiple fractioning by chromatography of the reaction resulting material. The puri Related U.S. Application Data fied polymer or dimer can be used as a colorant by itself or in (60) Provisional application No. 61/826.391, filed on May combination with another colorant for imparting color to a 22, 2013, provisional application No. 61/836,072, food, a drug, a cosmetic, a medical device, and textile prod filed on Jun. -

Colour Guide 2020 Index
COLOUR GUIDE 2020 INDEX COLOURS 1 - New colours SS20 Go to 2 - All colours Go to In this colour guide you will find the Pantone, CMYK and RGB Colour code references for the Stanley/Stella colour range. Stanley/Stella products are priced according to colour group: IMPORTANT Colour equivalences between TCX and C are provided by my 1 - Whites Pantone™ X-ref. CMYK and RGB references have been defined 2 - Colours to match our fabric colours as accurately as possible. 3 - Essential Heathers 4 - Special Heathers Certain colours (heathers, fluos, tie & dye and garment dyed 5 - All over prints (AOP) colours) are difficult to translate into accurate colour references. Colour Name - C000 - 1 If you have any doubts in relation to a colour we recommend that C 00 Pantone R 000 you order a sample. M 00 00-0000 TCX G 000 Y 00 0000 C B 00 Colour appearance may vary depending on the screen. K 00 2 NEW COLOURS - SS20 G-Dyed Fluo Juicy Melon - C044 - 2 G-Dyed Canyon-Pink - C512 - 2 C 00 C 12 Pantone R 255 Pantone R 198 M 65 M 43 16-1360 TCX G 132 16-1511 TCX G 155 Y 75 Y 33 811 C B 76 C512 B 149 K 00 K 06 G-Dyed Fluo Pink Crush - C042 - 2 Majorelle Blue - C034 - 2 C 00 C 97 Pantone R 255 Pantone R 34 M 63 M 62 16-1641 TCX G 122 19-4057 TCX G 75 Y 41 Y 06 805 C B 129 654 C B 133 K 00 K 22 Canyon Pink - C038 - 2 Heather Snow Mid Blue - C700 - 4 C 03 C 88 Pantone R 223 Pantone R 53 M 41 M 69 15-1614 TCX G 171 19-3936 TCX G 68 Y 27 Y 12 507 C B 166 2111 C B 117 K 00 K 29 Tie&Dye Majorelle Blue - C810 - 5 Tie&Dye Canyon Pink - C809 - 5 C 04 C 04 Pantone R 242 -

The Named Colors List Contains Over 1.500 Color Names with HEX and RGB Values, from "Absolute Zero" to "Zomp"
The Named Colors list contains over 1.500 color names with HEX and RGB values, from "Absolute Zero" to "Zomp". Color Preview Color Name Hex RGB Absolute Zero 0048BA 0, 72, 186 Acid green B0BF1A 176, 191, 26 Aero 7CB9E8 124, 185, 232 Aero blue C9FFE5 201, 255, 229 African violet B284BE 178, 132, 190 Air superiority blue 72A0C1 114, 160, 193 Alabama crimson AF002A 175, 0, 42 Alabaster F2F0E6 242, 240, 230 Aliceblue F0F8FF 240, 248, 255 Alloy orange C46210 196, 98, 16 Almond EFDECD 239, 222, 205 Aloeswood brown (Tonocha) 5A6457 90, 100, 87 Aloewood-color (Kyara-iro) 6A432D 106, 67, 45 Amaranth E52B50 229, 43, 80 Amaranth deep purple 9F2B68 159, 43, 104 Amaranth pink F19CBB 241, 156, 187 Amaranth purple AB274F 171, 39, 79 Amaranth red D3212D 211, 33, 45 Amazon 3B7A57 59, 122, 87 Amber FFBF00 255, 191, 0 Amber (Kohaku-iro) CA6924 202, 105, 36 Amber (SAE/ECE) FF7E00 255, 126, 0 Amethyst 9966CC 153, 102, 204 Amur cork tree (Kihada) F3C13A 243, 193, 58 Anti-flash white F2F3F4 242, 243, 244 Antique brass CD9575 205, 149, 117 Antique bronze 665D1E 102, 93, 30 Antique fuchsia 915C83 145, 92, 131 Color Preview ACnotlioqru eN arumbey H8e4x1B2D R1G32B, 27, 45 Antiquewhite FAEBD7 250, 235, 215 Apple 66B447 102, 180, 71 Apple green 8DB600 141, 182, 0 Apricot FBCEB1 251, 206, 177 Aqua 00FFFF 0, 255, 255 Aqua Blue color (Mizu-iro) 86ABA5 134, 171, 165 Aquamarine 7FFFD4 127, 255, 212 Arctic lime D0FF14 208, 255, 20 Army green 4B5320 75, 83, 32 Artichoke 8F9779 143, 151, 121 Arylide yellow E9D66B 233, 214, 107 Ash gray B2BEB5 178, 190, 181 Asparagus 87A96B -

Size Chart Unisex (Cm)
UNISEX CREW NECK SWEATSHIRTS CHANGER 20 per box STSU823 The iconic unisex crew neck sweatshirt Brushed Sweatshirt 85% Organic ring-spun combed cotton, 15% Recycled polyester 350 GSM . Set-in sleeve . 1x1 rib at neck collar, sleeve hem and bottom hem . Inside herringbone back neck tape . Self fabric half moon at back neck . Single needle topstitch at neck collar . Armhole, sleeve hem and bottom hem with twin needle topstitch WHITES FITTED XXS* XS S M L XL XXL XXXL* XXXXL* WHITE NATURAL RAW COLORS Size guide NEWNEW NEWNEW BLACK CAMEL DEEP CHOCOLATE CARAMEL MUSHROOM OCHRE GOLDEN YELLOW HAY YELLOW VARSITY GREEN C NEWNEW NEWNEW NEWNEW NEWNEW A 2.5cm below armhole B BOTTLE GREEN MOSS GREEN BRITISH KHAKI STEM GREEN TEAL MONSTERA SERENE BLUE SKY BLUE MAJORELLE BLUE INDIA INK GREY NEWNEW FRONT BACK LAVA GREY FRENCH NAVY BURGUNDY LAVENDER DAWN LILAC PETAL MAUVE COTTON PINK CANYON PINK TANGERINE SIZES XXS XS S M L XL XXL XXXL XXXXL A Half Chest 45.5 48 50.5 53 56 59 62 65 70 B Body Length 63 65 68 72 74 76 78 80 82 BRIGHT RED ROASTED ORANGE C Sleeve Length 61 62 64.5 66 67.5 69 70.5 70.5 70.5 ESSENTIAL HEATHERS Combo options HEATHER GREY MID HEATHER MID HEATHER DARK HEATHER KHAKI GREEN BLUE SPECIAL HEATHERS Mover Mini Changer HEATHER NEPPY HEATHER NEPPY LEMON GRASS BURGUNDY Printing Specifications *Size available on limited number of colours For suitable print techniques, please download our printing guide Please check our webshop for latest information Wash similar colours together, do not iron on print, wash and iron inside out.