Procainamide Hydrochloride 1333
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter And
J Am Board Fam Med: first published as 10.3122/jabfm.2011.01.080096 on 5 January 2011. Downloaded from CLINICAL REVIEW Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter and Fibrillation Madhuri Nair, MD, Lekha K. George, MD, and Santhosh K. G. Koshy, MD This article reviews the safety and efficacy of ibutilide for use in patients with atrial fibrillation and flut- ter. Ibutilide, a class III antiarrhythmic agent, is primarily used for conversion of atrial flutter and fi- brillation and is a good alternative to electrical cardioversion. Ibutilide has a conversion rate of up to 75% to 80% in recent-onset atrial fibrillation and flutter; the conversion rate is higher for atrial flutter than for atrial fibrillation. It is also safe in the conversion of chronic atrial fibrillation/flutter among patients receiving oral amiodarone therapy. Ibutilide pretreatment facilitates transthoracic defibrilla- tion and decreases the energy requirement of electrical cardioversion by both monophasic and biphasic shocks. Pretreatment with ibutilide before electrical defibrillation has a conversion rate of 100% com- pared with 72% with no pretreatment. Ibutilide is also safe and efficient in the treatment of atrial fibril- lation in patients who have had cardiac surgery, and in accessory pathway–mediated atrial fibrillation where the conversion rate of ibutilide is as high as 95%. There is up to a 4% risk of torsade de pointes and a 4.9% risk of monomorphic ventricular tachycardia. Hence, close monitoring in an intensive care unit setting is warranted during and at least for 4 hours after drug infusion. The anticoagulation strat- egy is the same as for any other mode of cardioversion.(J Am Board Fam Med 2011;24:86–92.) Keywords: Antiarrhythmics, Arrhythmia, Atrial Fibrillation, Cardiovascular Disorders, Cardioversion, Drug Ther- copyright. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -
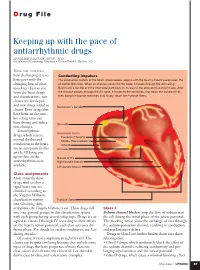
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Procainamide in the Treatment of Myotonia in Myotonic Dystrophy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.45.5.461 on 1 May 1982. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry 1982;45:461-463 Short report A comparative study of disopyramide and procainamide in the treatment of myotonia in myotonic dystrophy MICHAEL FINLAY From the Department ofNeurology, Royal Hallamshire Hospital, Sheffield SUMMARY Ten patients with myotonic dystrophy were allocated at random to treatment with disopyramide and procainamide in a cross-over trial. Disopyramide was found to be at least as effective as procainamide in the relief of myotonia; and two patients who could not tolerate procainamide both tolerated disopyramide. Myotonic dystrophy was first described by Steinert' trophy for between 4 and 21 years were included in the women and the clinical features have been reviewed by trial. There were seven men and three aged be- Protected by copyright. Thomasen2 and others. There have been many tween 31 and 59 years. All complained of weakness of their attempts to elucidate the underlying pathophysi- hands and the majority had noticed difficulty in relaxation of grip. Eight had also experienced impairment of gait. All ology. The basic abnormality has been ascribed to exhibited bilateral ptosis, weakness and wasting of the hypersensitivity of the muscle membrane,3 increased masseters, temporalis, facial and stemomastoid muscles membrane fluidity4 and impairment of normal and wasting and weakness of the forearms and hands. The neurotropic influences exerted by motor neurones seven men had similar distal wasting and weakness of the on muscle fibres.5 6 Quinine, steroids and pro- legs but in two women the lower limbs were symptomati- cainamide have all been used for treatment of the cally normal; in the other woman, there was proximal myotonia with variable success. -

PHARMACY TIMES by IEHP PHARMACEUTICAL SERVICES DEPARTMENT February 11, 2013
PHARMACY TIMES BY IEHP PHARMACEUTICAL SERVICES DEPARTMENT February 11, 2013 The Centers for Medicare and Medicaid Services (CMS) developed performance and quality measures to help Medicare beneficiaries make informed decisions regarding health and prescription drug plans. As part of this effort, CMS adopted measures for High Risk Medication (HRM) endorsed by the Pharmacy Quality Alliance (PQA) and the National Quality Forum (NQF). The HRM was developed using existing HEDIS measurement “Drugs to be avoided in the elderly”. The HRM rate analyzes the percentage of Medicare Part D beneficiaries 65 years or older who have received prescriptions for drugs with a high risk of serious side effects in the elderly. In order to advance patient safety, IEHP will be identifying members over 65 and currently on one of the medications identified in Table 1. Providers will be receiving a list of these members from IEHP on an ongoing basis. IEHP asks providers to review their member’s current drug regimen and safety risk and make any appropriate changes when applicable. Table 1: Medications identified by CMS to be high risk in the elderly: Drug Class Drug Safety Concerns IEHP Formulary Alternative(s) Acetylcholinesterase Donepezil (in patients Orthostatic hypotension or Memantine Inhibitor with syncope) bradycardia Amphetamines Dextroamphetamine CNS stimulation Weight Control: Diet Lisdextroamphetamine & lifestyle Diethylpropion modification Methylphenidate Phentermine Depression: mirtazapine, trazodone Analgesic Pentazocine Confusion, hallucination, Mild Pain: (includes Meperidine delirium, fall, fracture APAP combination Tramadol Lowers seizure threshold medications) Aspirin > 325 mg/day GI bleeding/peptic ulcer, edema Mod-Severe Pain Diflunisal may worsen heart failure Norco Etodolac Vicodin Fenoprofen Percocet Ketoprofen Morphine Meclofenamate Mefenamic acid Nabumetone 303 E. -

Get in Rhythm with the Safe and Effective Use of Antiarrythmic Drugs
Get in Rhythm with the Safe and Effective Use of Antiarrythmic Drugs Karen J. McConnell, Pharm.D., FCCP, BCPS- AQ Cardiology, ASH-CHC Clinical Director and Cardiology Subject Matter Expert, Cardinal Health Clinical Associate Professor, University of Colorado Skaggs School of Pharmacy and Shannon W. Finks, Pharm.D., FCCP, BCPS- AQ Cardiology, ASH-CHC Professor of Clinical Pharmacy, University of Tennessee College of Pharmacy Clinical Specialist Cardiology, Veterans Affairs Medical Center Memphis Disclosure The program chair and presenters for this continuing education activity have reported no relevant financial relationships. Objectives . Design patient-specific treatment and monitoring plans for antiarrhythmic drugs (AADs) to treat atrial fibrillation (AF) . Differentiate among appropriate monitoring strategies for various agents used in ventricular arrhythmia suppression . Avoid potential adverse drug events with AADs by identifying important drug interactions . Ensure safe and effective dosing of AADs based upon specific patient factors Rhythm Rule #1 . Pharmacists play a vital role in the appropriate use of AAD dosing, adverse effects, interactions, and monitoring. Treatment and Monitoring of Atrial Fibrillation Atrial Fibrillation • Most common type of serious arrhythmia • In U.S., affects 2-5 million patients • Frequently seen with comorbidities • AF complicates management of comorbidity • Comorbidity complicates management of AF • Associated with stroke, heart failure, death • Most common arrhythmia requiring hospitalization Case #1: Mary Rhythm 60 y/o AA woman with a PMH including . Laboratory data: HFrEF (EF 35%), atrial fibrillation(AF), CKD, 140 110 18 HTN 105 . Inpatient Medications: 4.7 22 1.6 apixaban 5 mg twice daily lisinopril 20 mg daily BP 115/78 mm Hg HT 67 in metoprolol succinate 50 mg/day HR 70 bpm WT 75 kg furosemide 40 mg twice daily spironolactone 25 mg/day . -

Drug Misuse and Dependence UK Guidelines on Clinical Management Drug Misuse and Dependence UK Guidelines on Clinical Management
Drug misuse and dependence UK guidelines on clinical management Drug misuse and dependence UK guidelines on clinical management Prepared by Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group Title: Drug misuse and dependence: UK guidelines on clinical management Recommended citation: Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group (2017) Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health Author: Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group Publisher: Global and Public Health / Population Health / Healthy Behaviours / 25460 Document purpose: Guidance Publication date: July 2017 (minor revisions November 2017) Target audience: Healthcare professionals Providers and commissioners of treatment for people who misuse or are dependent on drugs Professional and regulatory bodies Service users and carers Contact details: Alcohol, Drugs & Tobacco Division Public Health England [email protected] You may re-use the text of this document (not including logos) free of charge in any format or medium, under the terms of the Open Government Licence. To view this licence, visit www.nationalarchives.gov.uk/ doc/open-government-licence/ © Crown copyright Published to gov.uk www.gov.uk/dh Contents 1 Contents Preface 5 Professor Sir John Strang 5 Chapter 1: Introduction 9 Chapter 2: Essential elements of treatment provision 15 2.1 Key points 15 2.2 Assessment, planning care -

Objectives: to Compare "The Safety and Efficacy of Intravenous Procainamide and Amiodarone in the Acute Treatment of Wide Q
Critical Review Form PGY-1 Therapy Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J; PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2017 May 1;38(17):1329-1335. Objectives: To compare "the safety and efficacy of intravenous procainamide and amiodarone in the acute treatment of wide QRS complex monomorphic tachycardias (presumably VT) which are haemodynamically well tolerated." (p. 1330) Methods: This prospective, multicenter, randomized, open-label trial was conducted at 29 hospitals in Spain over a six-year period. Hemodynamically stable patients with tachycardia with a wide QRS complex who required medical attention were randomized to receive either IV procainamide (10 mg/kg over 20 minutes) or IV amiodarone (5 mg/kg over 20 minutes). Inclusion criteria were: 1. Regular heart rhythm with a rate ≥ 120 bpm 2. QRS ≥ 120 3. Systolic blood pressure ≥ 90 mmHg 4. Absence of dyspnea at rest 5. Absence of signs of peripheral hypoperfusion 6. Absence of severe anginal symptoms 7. Age > 18. Patients felt to have supraventricular tachycardia by physician criteria, those receiving IV amiodarone or procainamide in the prior 24 hours, and those with contraindications to the study drugs were excluded. The 40-minute "study period" was defined as the 20 minutes during which the drug was administered and 20 minutes following completion of administration. All patients were observed for 24 hours following study drug administration. The primary outcome was major cardiac adverse events, defined as any of the following 1) Clinical signs of peripheral hypoperfusion 2) Dyspnea at rest or orthopnea with signs of pulmonary congestion 3) Severe hypotension (SBP ≤ 70 if the pretreatment SBP was ≤ 100 or SBP ≤ 80 if the pretreatment SBP was > 100) 4) Acceleration of HR > 20 bpm of its mean value; or 5) development of polymorphic ventricular tachycardia. -

Procainamide for Wide Complex Tachycardia Pharmacology
Procainamide for Wide Complex Tachycardia Introduction 1. Ventricular tachycardia (VT) is an uncommon but dangerous medical condition, with an extremely variable clinical presentation. 2. Intravenous procainamide is guideline recommended and is the drug of choice for the treatment of hemodynamically stable VT with a class IIa recommendation. 3. Procainamide is an old drug with new evidence that supports it’s use but dosing strategies and administration techniques makes it difficult to use at the bedside. Pharmacology Procainamide Bolus Dosing • 10-17 mg/kg over 20-60 minutes (Max dose suggest 1g and max rate of 20-50 mg/min) or • 100 mg every 5 minutes at max rate of 50 mg/min to max dose 1g Dose and Renal Adjustments administration • eCrCl 10-50 ml/min: Reduce initial dosing by 25-50 % • eCrCL < 10 ml/min: Reduce initial dosing by 50-75% Maintenance Infusion Dosing • 1-6 mg/min Class 1A anti-arrhythmic that binds to fast sodium channels inhibiting recovery after Mechanism of • repolarization. It also prolongs the action potential and reduces the speed of impulse Action conduction • Onset: IV <2 minutes; IM 10-30 minutes • Time to Peak: IV 25-60 minute; IM 15-60 minutes • Duration: IV/IM: 3-4 hours PK/PD • Metabolism: Converted by the liver to N-acetylprocainamide (NAPA), an active compound • Half-life: 2.5– 4.7 hr (NAPA— 7 hr); increased in renal impairment • Excretion: 40– 70% excreted unchanged by the kidneys • Hypotension • Hepatotoxicity • Positive ANA titer Adverse Effects • Lupus-like syndrome • Anaphylaxis caused by sulfite salt -

Uptake of the Noncytotoxic Transport Probe Procainamide in the Chinese Hamster Ovary Model of Multidrug Resistance1
(CANCER RESEARCH 52. 3539-3546. July 1. 1992] Uptake of the Noncytotoxic Transport Probe Procainamide in the Chinese Hamster Ovary Model of Multidrug Resistance1 K. V. Speeg, Jr.,2 Catherine deLeon, and William L. McGuiret Department of Medicine, Divisions of Gastroenterologe/Nutrition [K. V. S., C. D.J and Oncology [W. L. M.], The University of Texas Health Science Center at San Antonio and Audie Murphy VA Hospital, San Antonio, Texas 78284 ABSTRACT to be a normal constituent of cells in organs known to secrete organic cations (i.e., renal proximal tubule, hepatocyte bile Many of the cytotoxic substrates of the multidrug transporter are canaliculus, and adrenal medulla) (7-9), we used noncytotoxic organic cations. Cimetidine, procainamide, and tetraethylammonium bro mide were used in a Chinese hamster ovary model of multidrug resistance, probes of organic cation secretion (10, 11), which is generally to study handling of noncytotoxic cationic transport probes. Cimetidine considered to be driven by electrochemical gradients rather and procainamide, but not tetraethylammonium, accumulated to a greater than by an ATPase (12), to determine whether these probes extent (5-fold) in the sensitive CHOAUXB1 (AB) cell line than in the might be substrates for gp-170 or whether this secretory mech resistant ( 11"( 5 (C5) cell line. Accumulation of both cimetidine and anism could be characterized in MDR cells. procainamide was significantly increased by verapamil in CS but not AB. Procainamide accumulation in both AB and CS was temperature depend ent and occurred by passive diffusion. Diltiazem, nifedipine, rifampin, MATERIALS AND METHODS tamoxifen, rhodamine, and ethidium also increased procainamide accu Materials. -

Cardiac Arrhythmias
Intensive Care Nursery House Staff Manual Neonatal Cardiac Arrhythmias INTRODUCTION: Identification and treatment of arrhythmias in newborns is challenging and differs from older children, and the natural history of arrhythmias presenting in the neonatal period is often dramatically different. METHODS OF DIAGNOSIS AND THERAPY: For management of arrhythmias, consult Cardiology team. 1. Diagnostic methods: •15 lead electrocardiogram (standard 12 lead plus V3R, V4R, V7) •Heart rate determination (ECG strip, count number of QRS complexes in 6 sec x 10) •Blood pressure (intra-arterial or indirect) 2. Treatment: Electrical (See below for drug therapy) •Artificial pacing : -Temporary transvenous pacing -Esophageal pacing •Cardioversion: -Setting: 0.5 - 2.0 Joules/kg -Mode: synchronous for narrow QRS; asynchronous for ventricular fibrillation IMMEDIATE MANAGEMENT OF ARRHYTHMIAS: Tachyarrhythmia Bradyarrhythmia narrow-QRS wide-QRS stable unstable stable unstable unstable vagal maneuvers synchronized Procainamide, synchronized A B C*, O2, cardioversion lidocaine in VT cardioversion adenosine, atropine & propranolol no response isoproterenol, or digoxin asynchronous temporary trans- mode venous pacing *A B C, airway, breathing, circulation 105 Copyright © 2004 The Regents of the University of California Neonatal Cardiac Arrhythmias A. Tachy-arrhythmias with narrow QRS: I. Reentry tachycardias Diagnosis Findings on ECG Treatment Atrial flutter -"Sawtooth" flutter waves -Unstable: esophageal pacing or -AV block does not terminate atrial rhythm electrical cardioversion -Atrial rate up to 500 in newborns -Variable AV conduction common -Stable: digoxin, propranolol, or digoxin + procainamide Accessory pathway mediated -P follows QRS, typically on upstroke of T tachycardia (WPW) -Superior or rightward P wave axis -AV block always terminates tachycardia -Unstable: esophageal pacing or -Typically terminates with P wave electrical cardioversion -After termination, WPW have pre-excitation -Stable: vagal maneuvers.