Transformation of the Recalcitrant Pesticide Chlordecone By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multi-Scale Impact of Chronic Exposure to Environmental Concentrations Of
Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta Romain Colpaert, Pierre-Henri Villard, Laetitia de Jong, Marina Mambert, Karim Benbrahim, Joelle Abraldes, Claire Cerini, Valérie Pique, Maxime Robin, Xavier Moreau To cite this version: Romain Colpaert, Pierre-Henri Villard, Laetitia de Jong, Marina Mambert, Karim Benbrahim, et al.. Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta. Environmental Science and Pollution Research, Springer Verlag, 2020, 27 (33), pp.41052-41062. 10.1007/s11356-019-06859-4. hal-02451113 HAL Id: hal-02451113 https://hal-amu.archives-ouvertes.fr/hal-02451113 Submitted on 23 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Multi-scale impact of chronic exposure to environmental concentrations of chlordecone in freshwater cnidarian, Hydra circumcincta. Romain COLPAERT1, Pierre-Henri VILLARD1, Laetitia DE JONG1, Marina MAMBERT1, Karim BENBRAHIM1, Joelle ABRALDES1, Claire CERINI2, Valérie PIQUE1, Maxime ROBIN1, Xavier MOREAU1 1 : Aix Marseille Univ, Avignon Univ, CNRS, IRD, IMBE, Marseille, France 2 : Aix Marseille Univ, Inserm U1263, C2VN, Marseille, France Corresponding author: email : [email protected] phone : +33-(0)4-91-83-56-38 Abstract Chlordecone (CLD) is an organochlorine pesticide widely used by the past to control pest insects in banana plantations in the French West Indies. -

OECD Environment Health and Safety Publications Series on Testing and Assessment No
OECD Environment Health and Safety Publications Series on Testing and Assessment No. 21 Detailed Review Paper Appraisal of Test Methods for Sex Hormone Disrupting Chemicals Environment Directorate ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT Paris May 2001 1 Also Published in the Series Testing and Assessment: No. 1, Guidance Document for the Development of OECD Guidelines for Testing of Chemicals (1993; reformatted 1995) No. 2, Detailed Review Paper on Biodegradability Testing (1995) No. 3, Guidance Document for Aquatic Effects Assessment (1995) No. 4, Report of the OECD Workshop on Environmental Hazard/Risk Assessment (1995) No. 5, Report of the SETAC/OECD Workshop on Avian Toxicity Testing (1996) No. 6, Report of the Final Ring-test of the Daphnia magna Reproduction Test (1997) No. 7, Guidance Document on Direct Phototransformation of Chemicals in Water (1997) No. 8, Report of the OECD Workshop on Sharing Information about New Industrial Chemicals Assessment (1997) No. 9 Guidance Document for the Conduct of Studies of Occupational Exposure to Pesticides During Agricultural Application (1997) No. 10, Report of the OECD Workshop on Statistical Analysis of Aquatic Toxicity Data (1998) No. 11, Detailed Review Paper on Aquatic Testing Methods for Pesticides and industrial Chemicals (1998) No. 12, Detailed Review Document on Classification Systems for Germ Cell Mutagenicity in OECD Member Countries (1998) No. 13, Detailed Review Document on Classification Systems for Sensitising Substances in OECD Member Countries 1998) No. 14, Detailed Review Document on Classification Systems for Eye Irritation/Corrosion in OECD Member Countries (1998) No. 15, Detailed Review Document on Classification Systems for Reproductive Toxicity in OECD Member Countries (1998) No. -

(A) Sources, Including As Appropriate (Provide Summary Information
UNEP/POPS/POPRC.1/4 Format for submitting pursuant to Article 8 of the Stockholm Convention the information specified in Annex E of the Convention Introductory information Name of the submitting Party/observer NGO Observer: Pesticide Action Network on behalf of the International POPs Elimination Network (IPEN) Contact details Clare Butler Ellis PhD, M.Inst.P, C.Env. Pesticide Action Network UK [email protected] Joseph DiGangi, PhD Environmental Health Fund +001-312-566-0985 [email protected] Chemical name Chlordecone Chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-octahydro-1,3,4-metheno-2H- cyclobuta[cd]pentalen-2-one CAS=143-50-0 Common trade names: GC 1189, Kepone, Merex Synonyms: Chlordecone, Chlordecone Kepone, Decachloroketone, Decachlorooctahydro-1,3,4-metheno-2H-cyclobuta(cd)pentalen-2-one, Decachloropentacyclo(5.3.0.0.0.0 2,6,4,10,5,9)decane-3-one, Decachlorotetracyclodecanone decachlorooctahydro- , Date of submission 27 January 2006 (a) Sources, including as appropriate (provide summary information and relevant references) (i) Production data: Quantity 1 “Chlordecone is no longer produced commercially in the United States. Between 1951 and 1975, approximately 3.6 million pounds (1.6 million kg) of chlordecone were produced in the United States (Epstein 1978). During this period, Allied Chemical Annex E information on chlordecone 1 UNEP/POPS/POPRC.1/4 Company produced approximately 1.8 million pounds (816,500 kg) of chlordecone at plants in Claymont, Delaware; Marcus Hook, Pennsylvania and Hopewell, Virginia. In 1974, because of increasing demand for chlordecone and a need to use their facility in Hopewell, Virginia, for other purposes, Allied Chemical transferred its chlordecone manufacturing to Life Sciences Products Company (EPA 1978b). -

Research Article Effect of Chlordecone on The
NorCal Open Access Publications Journal of Aquatic Research and NORCAL Marine Sciences OPEN ACCESS PUBLICATION Volume 2; Issue 2 Asifa KP and Chitra KC Research Article ISSN 2639-4618 Effect of Chlordecone on the Reproductive Potential of the Cichlid Fish, Pseudetroplus Maculatus (Bloch, 1795) Asifa KP, Chitra KC* Endocrinology and Toxicology Laboratory, Department of Zoology, University of Calicut, Kerala, India. *Corresponding author: Chitra KC, Endocrinology and Toxicology Laboratory, Department of Zoology, University of Calicut, Kerala, India. Email: [email protected] Received Date: 16 March, 2019; Accepted Date: 03 May, 2019; Published Date: 17 May, 2019 Abstract Introduction The present study was undertaken to evaluate that chlordecone, During the past few decades, large number of environmental an organochlorine pesticide, possessing estrogenic properties contaminants such as, pesticides, pharmaceutical drugs, plas- are known to influence the reproductive potential of the cich- tic products, natural phytochemicals etc. has been continu- lid fish, Pseudetroplus maculatus. Chlordecone at two sublethal ously released into various compartments of both terrestrial concentrations, 3.5 and 7µg/L, were exposed to fish for 4, 7, 15 and aquatic ecosystems, which are known to disrupt the en- and 30 days in order to evaluate the level of vitellogenin, main- docrine system of animals, including humans. Such chemicals taining the control groups. Vitellogenin concentrations were are called endocrine-disrupting chemicals (EDCs), which can measured in both male and female fishes by using indirect end- interfere with biosynthesis, transport, action, metabolism and points, such as alkali-labile phosphoprotein (ALP), total pro- removal of various hormones in the body resulting in varia- tein and calcium concentrations in the blood plasma. -

In Silico Pharmacodynamics, Toxicity Profile and Biological Activities of the Saharan Medicinal Plant Limoniastrum Feei
Brazilian Journal of Pharmaceutical Sciences Article http://dx.doi.org/10.1590/s2175-97902017000300061 In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei Ouahab Ammar* Department of Pharmacy, Faculty of Medical Sciences, University of Batna 2, Algeria In-silico study was performed to find the pharmacodynamics, toxicity profiles and biological activities of three phytochemicals isolated from Limoniastrum feei (Plumbagenaceae). Online pharmacokinetic tools were used to estimate the potential of Quercetin, kaempferol-3-O-β-D-glucopyranoside (astragalin) and quercitin-7-O-β-D-glucopyranoside as specific drugs. Then the prediction of potential targets of these compounds were investigated using PharmMapper. Auto-Dock 4.0 software was used to investigate the different interactions of these compounds with the targets predicted earlier. The permeability of quercetin s rule of five. Hematopoietic prostaglandin (PG) D׳ was found within the range stated by Lipinski synthase (HPGDS), farnesyl diphosphate synthetase (FPPS) and the deoxycytidine kinase (DCK) were potential targets for quercetin, astragalin and quercetin 7, respectively. Quercetin showed antiallergic and anti-inflammatory activity, while astragalin and quercetin 7 were predicted to have anticancer activities. The activity of Astragalin appeared to be mediated by FPPS inhibition. The inhibition of DCK was predicted as the anticancer mechanisms of quercetin 7. The compounds showed interesting interactions and satisfactory binding energies when docked into their targets. These compounds are proposed to have activities against a variety of human aliments such as allergy, tumors, muscular dystrophy, and diabetic cataracts. Keywords: Limoniastrum feei/pharmacokinetics. Limoniastrum feei/biological activity. Quercetin. Astragalin. Quercetin 7. Medicinal plants. Molecular docking. -

Download in English
UNITED NATIONS BC UNEP/CHW.12/5/Add.9 Distr.: General 10 July 2015 Original: English Conference of the Parties to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Twelfth meeting Geneva, 4–15 May 2015 Agenda item 4 (b) (i) Matters related to the implementation of the Convention: scientific and technical matters: technical guidelines Technical guidelines Technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with the pesticides aldrin, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, chlordane, chlordecone, dieldrin, endrin, heptachlor, hexachlorobenzene, lindane, mirex, pentachlorobenzene, perfluorooctane sulfonic acid, technical endosulfan and its related isomers or toxaphene or with hexachlorobenzene as an industrial chemical Note by the Secretariat At its twelfth meeting, the Conference of the Parties to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal adopted, in decision BC-12/3 on technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with persistent organic pollutants, the technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with the pesticides aldrin, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, chlordane, chlordecone, dieldrin, endrin, heptachlor, hexachlorobenzene, lindane, mirex, pentachlorobenzene, perfluorooctane sulfonic acid, technical endosulfan and its related isomers or toxaphene or with hexachlorobenzene as an industrial chemical, on the basis of the draft technical guidelines contained in document UNEP/CHW.12/INF/15. The technical guidelines referred to above were prepared by the Food and Agriculture Organization of the United Nations as lead organization for this work taking into account comments received from members of the small intersesssional working group on persistent organic pollutants wastes by 27 March 2015. -

Beating the Bugs: Roles of Microbial Biofilms in Corrosion
Beating the bugs: roles of microbial biofilms in corrosion The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Li, Kwan, Matthew Whitfield, and Krystyn J. Van Vliet. "Beating the bugs: roles of microbial biofilms in corrosion." Corrosion Reviews 321, 3-6 (2013); © 2013, by Walter de Gruyter Berlin Boston. All rights reserved. As Published https://dx.doi.org/10.1515/CORRREV-2013-0019 Publisher Walter de Gruyter GmbH Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/125679 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ Beating the bugs: Roles of microbial biofilms in corrosion Kwan Li∗,‡, Matthew Whitfield∗,‡, and Krystyn J. Van Vliet∗,† ∗Department of Materials Science and Engineering and †Department of Biological Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 USA ‡These author contributed equally to this work Abstract Microbiologically influenced corrosion is a complex type of environmentally assisted corrosion. Though poorly understood and challenging to ameliorate, it is increasingly appreciated that MIC accelerates failure of metal alloys, including steel pipeline. His- torically, this type of material degradation process has been treated from either an electrochemical materials perspective or a microbiological perspective. Here, we re- view the current understanding of MIC mechanisms for steel – particularly those in sour environments relevant to fossil fuel recovery and processing – and outline the role of the bacterial biofilm in both corrosion processes and mitigation responses. Keywords: biofilm; sulfate-reducing bacteria (SRB); microbiologically influenced cor- rosion (MIC) 1 Introduction Microbiologically influenced corrosion (MIC) can accelerate mechanical failure of metals in a wide range of environments ranging from oil and water pipelines and machinery to biomedical devices. -

Hal 89-106 Alimuddin Enzim
Microbial community of black band disease on infection ... (Ofri Johan) MICROBIAL COMMUNITY OF BLACK BAND DISEASE ON INFECTION, HEALTHY, AND DEAD PART OF SCLERACTINIAN Montipora sp. COLONY AT SERIBU ISLANDS, INDONESIA Ofri Johan*)#, Dietriech G. Bengen**), Neviaty P. Zamani**), Suharsono***), David Smith****), Angela Mariana Lusiastuti*****), and Michael J. Sweet******) *) Research and Development Institute for Ornamental Fish Culture, Jakarta **) Department of Marine Science and Technology, Faculty of Fisheries and Marine Science, Bogor Agricultural University ***) Research Center for Oceanography, The Indonesian Institute of Science ****) School of Biology, Newcastle University, NE1 7RU, United Kingdom *****) Center for Aquaculture Research and Development ******) Biological Sciences Research Group, University of Derby, Kedleston Road, Derby, DE22 1GB, United Kingdom (Received 19 March 2014; Final revised 12 September 2014; Accepted 10 November 2014) ABSTRACT It is crucial to understand the microbial community associated with the host when attempting to discern the pathogen responsible for disease outbreaks in scleractinian corals. This study determines changes in the bacterial community associated with Montipora sp. in response to black band disease in Indonesian waters. Healthy, diseased, and dead Montipora sp. (n = 3 for each sample type per location) were collected from three different locations (Pari Island, Pramuka Island, and Peteloran Island). DGGE (Denaturing Gradient Gel Electrophoresis) was carried out to identify the bacterial community associated with each sample type and histological analysis was conducted to identify pathogens associated with specific tissues. Various Desulfovibrio species were found as novelty to be associated with infection samples, including Desulfovibrio desulfuricans, Desulfovibrio magneticus, and Desulfovibrio gigas, Bacillus benzoevorans, Bacillus farraginis in genus which previously associated with pathogenicity in corals. -
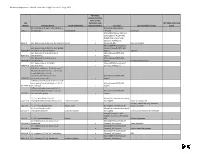
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

2019 Minnesota Chemicals of High Concern List
Minnesota Department of Health, Chemicals of High Concern List, 2019 Persistent, Bioaccumulative, Toxic (PBT) or very Persistent, very High Production CAS Bioaccumulative Use Example(s) and/or Volume (HPV) Number Chemical Name Health Endpoint(s) (vPvB) Source(s) Chemical Class Chemical1 Maine (CA Prop 65; IARC; IRIS; NTP Wood and textiles finishes, Cancer, Respiratory 11th ROC); WA Appen1; WA CHCC; disinfection, tissue 50-00-0 Formaldehyde x system, Eye irritant Minnesota HRV; Minnesota RAA preservative Gastrointestinal Minnesota HRL Contaminant 50-00-0 Formaldehyde (in water) system EU Category 1 Endocrine disruptor pesticide 50-29-3 DDT, technical, p,p'DDT Endocrine system Maine (CA Prop 65; IARC; IRIS; NTP PAH (chem-class) 11th ROC; OSPAR Chemicals of Concern; EuC Endocrine Disruptor Cancer, Endocrine Priority List; EPA Final PBT Rule for 50-32-8 Benzo(a)pyrene x x system TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota HRV WA Appen1; Minnesota HRL Dyes and diaminophenol mfg, wood preservation, 51-28-5 2,4-Dinitrophenol Eyes pesticide, pharmaceutical Maine (CA Prop 65; IARC; NTP 11th Preparation of amino resins, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development ROC); WA Appen1 solubilizer, chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP Research; PAH (chem-class) 11th ROC; EPA Final PBT Rule for 53-70-3 Dibenzo(a,h)anthracene Cancer x TRI; WA PBT List; OSPAR Chemicals of Concern); WA Appen1; Oregon P3 List Maine (CA Prop 65; NTP 11th ROC); Research 53-96-3 2-Acetylaminofluorene Cancer WA Appen1 Maine (CA Prop 65; IARC; IRIS; NTP Lubricant, antioxidant, 55-18-5 N-Nitrosodiethylamine Cancer 11th ROC); WA Appen1 plastics stabilizer Maine (CA Prop 65; IRIS; NTP 11th Pesticide (EPA reg. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Desulfovibrio Vulgaris Defenses Against Oxidative and Nitrosative Stresses
Desulfovibrio vulgaris defenses against oxidative and nitrosative stresses Mafalda Cristina de Oliveira Figueiredo Dissertation presented to obtain the Ph.D degree in Biochemistry Instituto de Tecnologia Química e Biológica | Universidade Nova de Lisboa Supervisor: Dr. Lígia M. Saraiva Co-supervisor: Prof. Miguel Teixeira Oeiras, September 2013 From left to right: Carlos Romão (president of the jury), Fernando Antunes (3rd opponent), Ana Melo (4th opponent), Lígia Saraiva (supervisor), Carlos Salgueiro (2nd opponent), Mafalda Figueiredo, Alain Dolla (1st opponent) and Miguel Teixeira (co-supervisor). 17th September 2013 Second edition, October 2013 Molecular Genetics of Microbial Resistance Laboratory Instituto de Tecnologia Química e Biológica Universidade Nova de Lisboa 2780-157 Portugal “Nothing in life is to be feared, it is only to be understood. Now is the time to understand more, so that we may fear less.” Marie Curie Acknowledgments The present work would not have been possible without the help, the support and the friendship of several people whom I would like to formally express my sincere gratitude: Dr. Lígia M. Saraiva Firstly I would like to express my gratitude to my supervisor Dr. Lígia M. Saraiva, without her ideas and persistence I would not have come this far. I thank her for the constant support and encouragement when things did not go so well, for the trust she placed in me and in my work and for always being there when I needed over these five years. I have to thank Dr. Lígia for the good advices and for the careful revision of this thesis. Thanks for everything!!! Prof. Miguel Teixeira To my co-supervisor Prof.