Biomechanical Effects of Different Vertebral Heights After
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2.20 Gansu Province
2.20 Gansu Province Gansu Provincial Prison Enterprise Group, affiliated with Gansu Provincial Prison Administration Bureau,1 has 18 prison enterprises Legal representative of the prison company: Liu Yan, general manager of Gansu Prison Enterprise Group2 His official positions in the prison system: Deputy director of Gansu Provincial Prison Administration Bureau No. Company Name of the Legal Person Legal Registered Business Scope Company Notes on the Prison Name Prison, to which and representative/ Title Capital Address the Company Shareholder(s) Belongs 1 Gansu Gansu Provincial Gansu Liu Yan 803 million Wholesale and retail of machinery 222 Jingning The Gansu Provincial Prison Provincial Prison Provincial Deputy director of yuan and equipment (excluding sedans), Road, Administration Bureau is Gansu Province’s Prison Administration Prison Gansu Provincial building materials, chemical Chengguan functional department that manages the Enterprise Bureau Administration Prison products, agricultural and sideline District, prisons in the entire province. It is in charge Group Bureau Administration products (excluding grain Lanzhou City of the works of these prisons. It is at the Bureau; general wholesale); wholesale and retail of deputy department level, and is managed by manager of Gansu daily necessities the Justice Department of Gansu Province.4 Prison Enterprise Group3 2 Gansu Dingxi Prison of Gansu Qiao Zhanying 16 million Manufacturing and sale of high-rise 1 Jiaoyu Dingxi Prison of Gansu Province6 was Dingqi Gansu Province Provincial Member of the yuan and long-span buildings, bridges, Avenue, established in May 1952. Its original name Steel Prison Communist Party marine engineering steel structures, An’ding was the Gansu Provincial Fourth Labor Structure Enterprise Committee and large boiler steel frames, District, Dingxi Reform Detachment. -

World Bank Document
Gansu Revitalization and Innovation Project: Procurement Plan Annex: Procurement Plan Procurement Plan of Gansu Revitalization and Innovation Project April 24, 2019 Public Disclosure Authorized Project information: Country: The People’s Republic of China Borrower: The People’s Republic of China Project Name: Gansu Revitalization and Innovation Project Loan/Credit No: Project ID: P158215 Project Implementation Agency (PIA): Gansu Financial Holding Group Co. Ltd (line of credit PPMO) will be responsible for microcredit management under Component 1. Gansu Provincial Culture and Tourism Department (culture and tourism PPMO) will be responsible for Component 2 and 3. The culture and Public Disclosure Authorized tourism PPMO will be centrally responsible for overseeing, coordinating, and training its cascaded PIUs at lower levels for subproject management. Both PPMOs will be responsible for liaison with the provincial PLG, municipal PLGs, and the World Bank on all aspects of project management, fiduciary, safeguards, and all other areas. The project will be implemented by eight project implementation units (PIUs) in the respective cities/districts/counties under the four prefecture municipalities. They are: Qin’an County Culture and Tourism Bureau, Maiji District Culture and Tourism Bureau, Wushan County Culture and Tourism Bureau, Lintao County Culture and Tourism Bureau, Tongwei County Culture and Tourism Bureau, Ganzhou District Culture and Tourism Bureau, Jiuquan City Culture and Tourism Bureau and Dunhuang City Culture and Tourism Bureau. Name of Components PIUs Gansu Financial Holding Group Co. Ltd (line of credit Public Disclosure Authorized PPMO). GFHG is designated as the wholesaler FI to handle Component 1. Under the direct oversight and Component 1: Increased Access to Financial management of the line of credit PPMO (GFHG), Bank Services for MSEs of Gansu is designated as the 1st participating financial institution (PFI) to handle micro- and small credit transactions. -

Study on Urban Efficiency Measurement and Spatiotemporal
sustainability Article Study on Urban Efficiency Measurement and Spatiotemporal Evolution of Cities in Northwest China Based on the DEA–Malmquist Model Jun Yin and Qingmei Tan * College of Economics and Management, Nanjing University of Aeronautics and Astronautics, Nanjing 211106, China; [email protected] * Correspondence: [email protected] Received: 18 November 2018; Accepted: 12 January 2019; Published: 15 January 2019 Abstract: Urban efficiency can effectively measure the management and allocation level of urban factor inputs. Based on the data of 30 prefecture-level cities in Northwest China from 2006 to 2015, urban efficiency is measured by data envelopment analysis (DEA). Then the spatiotemporal evolution rule is identified by Malmquist model. The results illustrate that the overall average urban efficiency of cities in Northwest China each year from 2006 to 2015 was at the low level. Only Jiayuguan, Yulin, Yan’an, and Karamay reached the high average urban efficiency, while Dingxi, Pingliang, Guyuan, Shangluo, Tianshui, Longnan, and Baiyin were at the inefficient level. Most cities in Northwest China were still in the “growing” stage of increasing returns to scale. The scale of urban investment was relatively insufficient, and economies of scale had not yet formed. Cities with decreasing returns to scale were mainly distributed in the capital cities and the central and sub-central cities of Guanzhong-Tianshui Economic Zone with relatively abundant urban resources and capital. Cities with constant returns to scale were mainly distributed in four cities including Yan’an, Yulin, Jiayuguan, and Karamay with high efficiency. The overall comprehensive efficiency, technical efficiency, and scale efficiency of cities in Northwest China were not only low, but also showing a downward trend. -
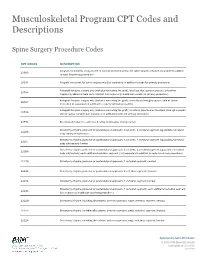
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020)
Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020) Procedure Code Description ACL Repair 27407 Repair, primary, torn ligament and/or capsule, knee; cruciate ACL Repair 27409 Repair, primary, torn ligament and/or capsule, knee; collateral and cruciate ligaments ACL Repair 29888 Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction Acromioplasty and Rotator Cuff Repair 23130 Acromioplasty Or Acromionectomy, Partial, With Or Without Coracoacromial Ligament Release Acromioplasty and Rotator Cuff Repair 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Acromioplasty and Rotator Cuff Repair 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Acromioplasty and Rotator Cuff Repair 23415 Coracoacromial Ligament Release, With Or Without Acromioplasty Acromioplasty and Rotator Cuff Repair 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Arthroscopy, Shoulder, Surgical; Decompression Of Subacromial Space With Partial Acromioplasty, With Coracoacromial Ligament (Ie, Arch) Release, When Performed (List Separately In Addition Acromioplasty and Rotator Cuff Repair 29826 To Code For Primary Procedure) Acromioplasty and Rotator Cuff Repair 29827 Arthroscopy, shoulder, surgical; with rotator cuff repair Allograft for Spinal Fusion [BMP] 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only Ankle Fusion 27870 Arthrodesis, ankle, open Ankle Fusion -

Evaluation of Green Development Efficiency of the Major Cities In
sustainability Article Evaluation of Green Development Efficiency of the Major Cities in Gansu Province, China Rongrong Liu 1,* , Dong Chen 2, Suchang Yang 1 and Yang Chen 3 1 School of Economics, Lanzhou University, Lanzhou 730000, China; [email protected] 2 Economic Management College of Agriculture and Forestry, Lanzhou University of Finance and Economics, Lanzhou 730101, China; [email protected] 3 School of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] * Correspondence: [email protected] Abstract: Green development (GD) has become a new model of sustainable development across the world. However, our knowledge of green development efficiency (GDE) in Gansu province is poor. In remedy, this study, based on the panel data of 12 major cities in Gansu from 2010 to 2017, employed the super-efficient Slack-based measure (SBM) to analyze and evaluate GDE from the input–output perspective. Furthermore, we analyzed the input redundancy and output deficiency of identified inefficient cities in 2017 and conducted spatial autocorrelation analysis of GDE of the cities under study. Results show differences in the GDE of the major cities in Gansu, with an average value of 0.985. Green development efficiency in Lanzhou, Qingyang, Jinchang, Jiuquan, and Tianshui was relatively higher than in other cities. Green development efficiency in Zhangye, Wuwei, Jiayuguan, Baiyin, Dingxi, Longnan, and Longnan was less than one due to their redundant labor and capital input and excessive pollutant emission output. The overall GDE in Gansu depicts “high east and low west” zones. Each city in Gansu needs to formulate targeted policies and regulations to improve Citation: Liu, R.; Chen, D.; Yang, S.; resource utilization, innovation capacity, reduce pollutant emission, optimize the industrial structure, Chen, Y. -

Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy
Y Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines 1 G Evidence-Based Clinical Guidelines for Multidisciplinary ETHODOLO Spine Care M NE I DEL I U /G ON Diagnosis and Treatment of I NTRODUCT Lumbar Disc I Herniation with Radiculopathy NASS Evidence-Based Clinical Guidelines Committee D. Scott Kreiner, MD Paul Dougherty, II, DC Committee Chair, Natural History Chair Robert Fernand, MD Gary Ghiselli, MD Steven Hwang, MD Amgad S. Hanna, MD Diagnosis/Imaging Chair Tim Lamer, MD Anthony J. Lisi, DC John Easa, MD Daniel J. Mazanec, MD Medical/Interventional Treatment Chair Richard J. Meagher, MD Robert C. Nucci, MD Daniel K .Resnick, MD Rakesh D. Patel, MD Surgical Treatment Chair Jonathan N. Sembrano, MD Anil K. Sharma, MD Jamie Baisden, MD Jeffrey T. Summers, MD Shay Bess, MD Christopher K. Taleghani, MD Charles H. Cho, MD, MBA William L. Tontz, Jr., MD Michael J. DePalma, MD John F. Toton, MD This clinical guideline should not be construed as including all proper methods of care or excluding or other acceptable methods of care reason- ably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment is to be made by the physi- cian and patient in light of all circumstances presented by the patient and the needs and resources particular to the locality or institution. I NTRODUCT 2 Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines I ON Financial Statement This clinical guideline was developed and funded in its entirety by the North American Spine Society (NASS). All participating /G authors have disclosed potential conflicts of interest consistent with NASS’ disclosure policy. -

Indications for Lumbar Total Disc Replacement: Selecting the Right Patient with the Right Indication for the Right Total Disc
Indications for Lumbar Total Disc Replacement: Selecting the Right Patient with the Right Indication for the Right Total Disc Karin Büttner-Janz, Dr.med., Prof., Richard D. Guyer and Donna D. Ohnmeiss, Dr.Med. Int J Spine Surg 2014, 8 () doi: https://doi.org/10.14444/1012 http://ijssurgery.com/content/8/12 This information is current as of September 24, 2021. Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://ijssurgery.com/alerts The International Journal of Spine Surgery 2397 Waterbury Circle, Suite 1, Aurora, IL 60504, Phone: +1-630-375-1432 © 2014 ISASS. All RightsDownloaded Reserved. from http://ijssurgery.com/ by guest on September 24, 2021 This article generously published free of charge by the International Society for the Advancement of Spine Surgery. Downloaded from http://ijssurgery.com/ by guest on September 24, 2021 Indications for Lumbar Total Disc Replacement: Selecting the Right Patient with the Right Indication for the Right Total Disc Karin Büttner-Janz, Dr.med., Prof.,1 Richard D. Guyer, MD,2 Donna D. Ohnmeiss, Dr.Med.3 1Büttner-Janz Spinefoundation, Berlin, Germany; 2Texas Back Institute, Plano, Texas; 3Texas Back Institute Research Foundation, Plano, Texas Abstract Summary of Background Data As with any surgery, care should be taken to determine patient selection criteria for lumbar TDR based on safety and optimizing outcome. These goals may initially be addressed by analyzing biomechanical implant function and early clinical experience, ongoing evaluation is needed to refine indications. Objective The purpose of this work was to synthesize information published on general indications for lumbar TDR. -

Commercial Musculoskeletal Codes
Updated January 2018 Commercial Musculoskeletal Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery Codes associated with an Arthrogram CPT Description Commercial Notes Partial excision of posterior vertebral component (eg, spinous 22100 process, lamina or facet) for intrinsic bony lesion, single vertebral segment; cervical 22101 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; thoracic 22102 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; lumbar Partial excision of posterior vertebral component (eg, spinous process, 22103 lamina or facet) for intrinsic bony lesion, single vertebral segment; each additional segment (List separately in addition to code for primary procedure) Partial excision of vertebral body, for intrinsic bony lesion, without 22110 decompression of spinal cord or nerve root(s), single vertebral segment;cervical Partial excision of vertebral body, for intrinsic bony lesion, without 22112 decompression of spinal cord or nerve root(s), single vertebral segment; thoracic Partial excision of vertebral body, for intrinsic bony lesion, without 22114 decompression of spinal cord or nerve root(s), single vertebral segment; lumbar each additional vertebral segment (list separately in addition to code 22116 for primary procedure) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 22206 1 vertebral