Revised Phylogeny and Historical Biogeography of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
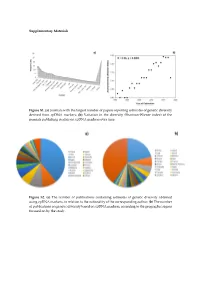
(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K. -

Hybridization Dynamics of Invasive Cattail (Typhaceae) Stands at Pierce Cedar Creek Institute: a Molecular Analysis
HYBRIDIZATION DYNAMICS OF INVASIVE CATTAIL (TYPHACEAE) STANDS AT PIERCE CEDAR CREEK INSTITUTE: A MOLECULAR ANALYSIS Alex Graeff, Kelsey Huisman, and Dr. Pamela J. Laureto Department of Biological Sciences Grand Rapids Community College 143 Bostwick NE Grand Rapids, Michigan 49503 ABSTRACT Three cattail taxa are recognized in Michigan USA: native Typha latifolia (broad-leaf cattail), the invasive Typha angustifolia (narrow-leaf cattail), and the hybrid of these two species Typha × glauca. Typha angustifolia and T. × glauca are of special interest because of their ability to aggressively spread and out-compete the native cattail T. latifolia. Typha × glauca has been shown to out-compete both its parental taxa and produce monospecific stands. We surveyed the Pierce Cedar Creek Institute (PCCI) property for cattails and located 25 distinct cattail marshes. We determined the total area of cattail marsh at PCCI to be roughly 10% of the 267 ha property. Cattail individuals were sampled from each of the 25 stands and random amplified polymorphic DNA markers were used to identify the individuals to species. We found that 20 of the 25 stands were monospecific for the native cattail, T. latifolia. Five of the stands were mixtures of the native T. latifolia and the introduced T. angustifolia, and T. × glauca was found in two of the mixed stands. We recommend removal of the invasive T. angustifolia and T. × glauca individuals and the establishment of a monitoring plan in order to maintain the long-term health of the cattail marshes at PCCI. Keywords: Typha spp., RAPD markers, invasive species 1 INTRODUCTION Species of Typha L. (Typhaceae), commonly known as cattails, are highly productive emergent plants that grow in a variety of wetland habitats throughout the world (McManus et al. -

Washington State Noxious Weeds
Changes to the 2014 Noxious Weed List Wendy DesCamp November 7, 2013 Today’s talk • Weed law review • New species for the 2014 noxious weed list • Other changes for 2014 to noxious weed list Noxious Weed • “Noxious weed” means a plant that when established is highly destructive, competitive, or difficult to control by cultural or chemical practices. RCW 17.10.10 The Noxious Weed Laws • RCW 17.10 – Limit economic loss due to the presence and spread of noxious weeds – Holds landowners responsible for controlling noxious weeds on their property – Noxious Weed Control Boards—county and state • RCW 17.04 and RCW 17.06 – Weed Districts The Noxious Weed Laws • WAC 16.750 – Weed list and schedule of penalties • WAC 16.752 – Prohibited plants, aka the quarantine list Noxious Weeds • Plants are noted as aggressive and highly difficult to control • Plants have a significant ecological impact, economic impact and/or cause harm to humans and other animals • 3 class of noxious weeds Class A Noxious Weeds • Class A consists of those noxious weeds – not native to the Washington – of limited distribution or are unrecorded in the state and – that pose a serious threat to the state • Eradication is required of all Class A noxious weeds • Currently 41 species Garlic mustard (Alliaria petiolata) Class B Noxious Weeds Scotch broom (Cytisus scoparius) • Class B: not native to the state and are of limited distribution or are unrecorded in a region of the state and that pose a serious threat to that region. • "Class B designate" means those Class B noxious weeds whose populations in a region or area are such that all seed production can be prevented within a calendar year. -

Differential Nitrogen and Phosphorus Recovery by Five Aquatic Garden
JOBNAME: horts 43#3 2008 PAGE: 1 OUTPUT: April 23 09:13:23 2008 tsp/horts/163067/02647 HORTSCIENCE 43(3):868–874. 2008. amount of pollutant that a water body can receive from point and nonpoint sources and still maintain its designated use and value Differential Nitrogen and Phosphorus (e.g., drinking water, fish and wildlife habitat, recreation, and so on). The Clean Water Act Recovery by Five Aquatic Garden (U.S. EPA, 1994) lists nitrogen (N) and phosphorus (P) as potential pollutants of Species in Laboratory-scale impaired water bodies. Offsite movement of – nitrate–nitrogen (NO3 ) and soluble reactive – 2– 3– phosphate (H2PO4 , HPO4 , and PO4 ) from Subsurface-constructed Wetlands nursery and greenhouse operations may lead Robert F. Polomski1,6, Douglas G. Bielenberg3, and Ted Whitwell5 to excessive algal and aquatic plant growth Department of Horticulture, Clemson University, 254 Poole Agricultural in surface waters, resulting in accelerated eutrophication. In general, freshwater systems Center, Clemson, SC 29634-0319 are P-limited and more prone to P inputs, Milton D. Taylor2 whereas N often limits primary production in estuarine and marine environments InsectiGen, Inc., 425 River Road, Athens, GA 30602 (Carpenter et al., 1998). 4 The maximum contaminant level for William C. Bridges – –1 NO3 in drinking water is 10 mgÁL Department of Applied Economics and Statistics, Clemson University, (National Academy of Sciences, 1977). No Clemson, SC 29634-0313 federal limits on P contamination in fresh- 4 water have been established as a result of Stephen J. Klaine variations in size, hydrology, and depth of Department of Biological Sciences, Clemson Institute of Environmental rivers and lakes and regional differences in Toxicology, P.O. -

Flora and Vegetation of Wadi El-Natrun Depression, Egypt
PHYTOLOGIA BALCANICA 21 (3): 351 – 366, Sofia, 2015 351 Habitat diversity and floristic analysis of Wadi El-Natrun Depression, Western Desert, Egypt Monier M. Abd El-Ghani, Rim S. Hamdy & Azza B. Hamed Department of Botany and Microbiology, Faculty of Science, Cairo University, Giza 12613, Egypt; email: [email protected] (corresponding author) Received: May 18, 2015 ▷ Accepted: October 15, 2015 Abstract. Despite the actual desertification in Wadi El-Natrun Depression nitrated by tourism and overuse by nomads, 142 species were recorded. Sixty-one species were considered as new additions, unrecorded before in four main habitats: (1) croplands (irrigated field plots); (2) orchards; (3) wastelands (moist land and abandoned salinized field plots); and (4) lakes (salinized water bodies). The floristic analysis suggested a close floristic relationship between Wadi El-Natrun and other oases or depressions of the Western Desert of Egypt. Key words: biodiversity, croplands, human impacts, lakes, oases, orchards, wastelands Introduction as drip, sprinkle and pivot) are used in the newly re- claimed areas, the older ones follow the inundation Wadi El-Natrun is part of the Western (Libyan) Desert type of irrigation (Soliman 1996; Abd El-Ghani & El- adjacent to the Nile Delta (23 m below sea level), lo- Sawaf 2004). Thus the presence of irrigation water as cated approximately 90 km southwards of Alexandria underground water of suitable quality, existence of and 110 km NW of Cairo. It is oriented in a NW–SE natural fresh water springs and availability of water direction, between longitudes 30°05'–30°36'E and lat- contained in the sandy layers above the shallow wa- itudes 30°29'–30°17'N (King & al. -

Aquatic Vascular Plants of New England, Station Bulletin, No.517
University of New Hampshire University of New Hampshire Scholars' Repository NHAES Bulletin New Hampshire Agricultural Experiment Station 2-1-1981 Aquatic vascular plants of New England, Station Bulletin, no.517 Crow, G. E. Hellquist, C. B. New Hampshire Agricultural Experiment Station Follow this and additional works at: https://scholars.unh.edu/agbulletin Recommended Citation Crow, G. E.; Hellquist, C. B.; and New Hampshire Agricultural Experiment Station, "Aquatic vascular plants of New England, Station Bulletin, no.517" (1981). NHAES Bulletin. 478. https://scholars.unh.edu/agbulletin/478 This Text is brought to you for free and open access by the New Hampshire Agricultural Experiment Station at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in NHAES Bulletin by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. S ON BULLETIN 517 February, 1981 oo-5n Aquatic Vascular Plants of Ne^v England: Part 2. Typhaceae and Sparganiaceae by G. E. Crow and C. B. Hellquist NEW HAMPSHIRE AGRICULTURAL EXPERIMENT STATION UNIVERSITY OF NEW HAMPSHIRE DURHAM, NEW HAMPSHIRE ON BULLETIN 517 February, 1981 Aquatic Vascular Plants of New England: Part 2. Typhaceae and Sparganiaceae by G. E. Crow and C. B. Hellquist NEW HAMPSHIRE AGRICULTURAL EXPERIMENT STATION UNIVERSITY OF NEW HAMPSHIRE DURHAM, NEW HAMPSHIRE •' Nst; Hrirr-poliire Y •'_v--r-r 5 %^ ACKNOWLEDGEMENTS ^^ 5/:^ We wish to thank Drs. Ernest O. Beal, Vernon L. Harms, Arthur C. Mathieson, and Eugene C. Ogden for their helpful comments on the manuscript. We are also grateful to the curators of the following her- baria for use of their collections: BOSC, BRU, CONN, CUW, GH, HNH, KIRI, MASS, MAINE, NCBS, NHA, NEBC, VT, YU. -

2. TYPHA Linnaeus, Sp. Pl. 2: 971. 1753. 香蒲属 Xiang Pu Shu Herbs, Perennial, in Marshes Or Aquatic, with Creeping Rhizomes
Fl. China 23: 161–163. 2010. 2. TYPHA Linnaeus, Sp. Pl. 2: 971. 1753. 香蒲属 xiang pu shu Herbs, perennial, in marshes or aquatic, with creeping rhizomes. Leaves alternate, erect, distichous, linear, usually spongy, mar- gin entire, sheathed at base. Flowers unisexual, minute, numerous, densely crowded in a cylindric spike with lower part female and upper part male; bracts leaflike. Perianth absent. Male flowers consisting of 1–3 stamens usually connate at base of filaments, sur- rounded by hairs; anthers 2-thecous, basifixed, longitudinally dehiscent; filaments short; pollen grains in monads or tetrads. Female flowers: ovary 1-loculed, on a long capillary stalk with many fine hairs or bracteoles at base; styles capillary; stigmas broadened or spatulate; ovule 1; sterile ovary without style. Fruit minute, falling off together with stalk. About 16 species: tropical and temperate regions; 12 species (three endemic) in China. 1a. Female flowers without bracteoles; female part of spikes connected with or separated from male part. 2a. Female part of spikes not separated from male part. 3a. Stigmas spatulate; hairs on stalk of female flowers ca. as long as style ......................................................... 1. T. orientalis 3b. Stigmas lanceolate; hairs on stalk of female flowers shorter than style ............................................................. 2. T. latifolia 2b. Female part of spikes separated from male part. 4a. Stigmas linear, slender; axis of male part in spikes with brown hairs, hairs furcate or not ......................... 3. T. przewalskii 4b. Stigmas spatulate; axis of male part in spikes with whitish or yellowish brown hairs, hairs not furcate. 5a. Stems 1.5–2 m tall; hairs on stalk of female flowers shorter than style .......................................... -

Taxonomy Monocots
Taxonomy Monocots- 1. Typhaceae - commonly called the Cattail Family (aceae ending means family). These are emergent, rhizomatons, found in fresh or brackish waters. • Typha (genus) domingensis (species): This is the species found in AZ. • Typha latifolia 2. Potamogetonaceae - the Pondweed Family. This family is rooted and submerged. • Potamogeton: commonly known as Pondweeds; many species are found. • Ruppia: commonly known as Widgeon grass; found in fresh or brackish waters. • Zannichelia: commonly known as Horned Pondweed; found in fresh or brackish waters. • Zoestra: marine seagrass. • Halodule: marine seagrass. • Cymodocea: marine seagrass. • Phyllospadix: marine seagrass. 3. Najadaceae - the Niad Family. This family is also rooted and submerged; there is only one genus. • Najas marina: commonly known as the spiny niad; found in brackish waters. Typically known as a problem plant because it grows course and very quickly. 4. Hydrocharitaceae - the Frogbit Family. This family is rooted and submerged, and is found in fresh and marine waters. • Anacharis densa: commonly known as Waterweed, also called Elodea. A very common aquarium plant, considered a problem plant in freshwater lakes. • Halophila: found in marine habitats. • Thalassia: commonly known as Turtlegrass (another type of seagrass); found in marine habitats. • Vallisneria: commonly known as Wild Celery, a common food for ducks and other water fowl; found in freshwater. 5. Graminaceae (Poaceae)- the Grass Family. Grasses can be identified by the swollen base of each leaf where it meets the stem. This is called a ligule. There are 22 genera, important ones are listed. Most of these are emergent and rooted. • Phragmites australis: commonly known as the Giant Reed, similar to Arundo; found in freshwater. -

Monographie Der Gattung Typha Tourn. (Typhinae Agdh., Typhaceae Schur-Engl.)
© Zool.-Bot. Ges. Österreich, Austria; download unter www.biologiezentrum.at 89 Monographie der Gattung Typha Tourn. (Typhinae Agdh., Typhaceae Schur-Engl.). Von Dr. M. Kronfeld. (Mit Tafel IV und V.) (Vorgelegt in der Versammlung am 7. Jänner 1888.) I. Einleitung. (Geschichtlicher Ueberblick. Allgemeines.) Es konnte nicht fehlen, dass eine allerwärts verbreitete und physiog- nomisch so sehr auffallende Gattung wie Typha schon von den ersten Natur- forschern beachtet wurde. In der That nennt bereits des Aristoteles Schüler Theophrast (371—286 n. Chr.) an mehreren Stellen seiner Pflanzengeschichte ein Gewächs ncpr). Und sicher ist, dass wir mindestens ein oder zwei der Beleg- stellen auf die Typha im heutigen Sinne beziehen dürfen. Legen wir nämlich die Ausgabe Theophrast's von Heinsius (Leyden, 1613) zu Grunde, so sind von vorneherein auszuschliessen: I 9, II 5, VIII 8 (p. 11, 31, 152, 155, 157), wo es sich bestimmt um ein gesätes (VIII) und in Weizen (II 5, VIII 8) umwandelbares Getreide1) handelt. Dagegen könnte die tup») I 8 mit knotenlosen Stengeln unsere Typha bedeuten. Ohne Zweifel ist als solche die Pflanze in IV 11 anzusehen. Von dieser heisst es, sie wachse im See Orchomenos und sei zur Speise geeignet, indem die Knaben gerne an dem zarten Theile kauen, welcher den Wurzeln zunächst ist. Des Näheren auf den ökonomischen Abschnitt verweisend, wollen wir hier nur daran erinnern, dass den Kosaken am Don der Wurzelstock von Typha latifolia noch heute zur beliebten Speise dient. Ob Theophrast diese Art im Sinne habe, wie Spren- gel2) meint, oder ob unter seiner xtcprj die in ganz Griechenland vorkommende Typha angustata Bory et Chaub. -

Arbuscular Mycorrhizal Fungi and Dark Septate Fungi in Plants Associated with Aquatic Environments Doi: 10.1590/0102-33062016Abb0296
Arbuscular mycorrhizal fungi and dark septate fungi in plants associated with aquatic environments doi: 10.1590/0102-33062016abb0296 Table S1. Presence of arbuscular mycorrhizal fungi (AMF) and/or dark septate fungi (DSF) in non-flowering plants and angiosperms, according to data from 62 papers. A: arbuscule; V: vesicle; H: intraradical hyphae; % COL: percentage of colonization. MYCORRHIZAL SPECIES AMF STRUCTURES % AMF COL AMF REFERENCES DSF DSF REFERENCES LYCOPODIOPHYTA1 Isoetales Isoetaceae Isoetes coromandelina L. A, V, H 43 38; 39 Isoetes echinospora Durieu A, V, H 1.9-14.5 50 + 50 Isoetes kirkii A. Braun not informed not informed 13 Isoetes lacustris L.* A, V, H 25-50 50; 61 + 50 Lycopodiales Lycopodiaceae Lycopodiella inundata (L.) Holub A, V 0-18 22 + 22 MONILOPHYTA2 Equisetales Equisetaceae Equisetum arvense L. A, V 2-28 15; 19; 52; 60 + 60 Osmundales Osmundaceae Osmunda cinnamomea L. A, V 10 14 Salviniales Marsileaceae Marsilea quadrifolia L.* V, H not informed 19;38 Salviniaceae Azolla pinnata R. Br.* not informed not informed 19 Salvinia cucullata Roxb* not informed 21 4; 19 Salvinia natans Pursh V, H not informed 38 Polipodiales Dryopteridaceae Polystichum lepidocaulon (Hook.) J. Sm. A, V not informed 30 Davalliaceae Davallia mariesii T. Moore ex Baker A not informed 30 Onocleaceae Matteuccia struthiopteris (L.) Tod. A not informed 30 Onoclea sensibilis L. A, V 10-70 14; 60 + 60 Pteridaceae Acrostichum aureum L. A, V, H 27-69 42; 55 Adiantum pedatum L. A not informed 30 Aleuritopteris argentea (S. G. Gmel) Fée A, V not informed 30 Pteris cretica L. A not informed 30 Pteris multifida Poir. -

Photographs Covering Western Desert, Eastern Desert, Sinai Peninsula, Nile Region
Appendix: Photographs Covering Western Desert, Eastern Desert, Sinai Peninsula, Nile Region A. Western Desert Photo A.1 A community dominated by the psammophyte Ammophila arenaria inhabiting the coastal sand dunes of the Western Mediterranean Coast, Egypt 375 376 Appendix Photo A.2 Salt marsh vegetation with abundant growth of Kochia indica (Bassia indica) in the foreground. Mixed halophytes of Juncus rigidus and Arthrocnemum macrostachyum in the background, Western Mediterranean Coast, Egypt Photo A.3 Dense growth of Juncus rigidus in the salt marshes of Siwa Oasis, Western Desert, Egypt Appendix 377 Photo A.4 Reed swamp vegetation dominated by Typha domingensis, Siwa Oasis, Western Desert, Egypt 378 Appendix Photo A.5 A Populus euphratica tree inhabiting a sand dune in Siwa Oasis, Western Desert, Egypt. A clump of Stipagrostis scoparia is seen in the foreground Appendix 379 Photo A.6 Dense stand dominated by Typha elephantina, Um Rishe Lake, Wadi El-Natrun Depression, Western Desert, Egypt Photo A.7 A close up view of the succulent xerophyte Zygophyllum coccineum, Cairo-Alexandria desert road, Western Desert, Egypt 380 Appendix Photo A.8 Pancratium sickenbergeri bulbous herb, Mariut Plateau, northern section of the Western Desert, Egypt Photo A.9 Close-up view of the annual herb Asphodelus tenuifolius growing in the Western Mediterranean Coast, northern section of the Western Desert, Egypt Appendix 381 B. Eastern Desert Photo A.10 Mangal vegetation dominated by Avicennia marina, Red Sea Coast, Egypt Photo A.11 Dense mangrove forest dominated by Rhizophora mucronata, Southern section of the Red Sea Coast, Egypt 382 Appendix Photo A.12 A close up view of Rhizophora mucronata mangrove tree, Shalateen swamps, southern section of the Red Sea Coast, Egypt Photo A.13 Mangrove swamp of Rhizophora mucronata with a seedling in the forgroung, Mersa Abu Fissi, Red Sea Coast, Egypt Appendix 383 Photo A.14 A general view of the mangrove forest lining the shore-line of Mersa Abu Fissi, Red Sea coast, Egypt.