Angiogenesis and the Ischaemic Heart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All V Integrins
Cell, Vol. 95, 507–519, November 13, 1998, Copyright ©1998 by Cell Press Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All ␣v Integrins Bernhard L. Bader,*‡ Helen Rayburn,* their ligands. Significant expression of ␣v integrins has Denise Crowley,* and Richard O. Hynes*† been noted, in particular, in neural crest cells (Delannet * Howard Hughes Medical Institute et al., 1994), glial cells (Hirsch et al., 1994; Milner and Center for Cancer Research ffrench-Constant, 1994), muscle (Hirsch et al., 1994; Mc- and Department of Biology Donald et al., 1995; Martin and Sanes, 1997), osteoclasts Massachusetts Institute of Technology (Va¨ a¨ na¨ nen and Horton, 1995), epithelia (␣v6; Breuss et Cambridge, Massachusetts 02139 al., 1995; Huang et al., 1996), and blood vessels during development (Brooks et al., 1994a; Drake et al., 1995; Friedlander et al., 1995, 1996) or angiogenesis in re- Summary sponse to tumors (Brooks et al., 1994a, 1994b, 1996, 1998; Varner et al., 1995). ␣v integrins have been implicated in many develop- Among the ligands for various ␣v integrins is fibro- mental processes and are therapeutic targets for inhi- nectin (FN), and results on mouse embryos lacking FN bition of angiogenesis and osteoporosis. Surprisingly, or FN receptor integrins suggest that ␣v integrins might ablation of the gene for the ␣v integrin subunit, elimi- be important receptors for FN during early development. nating all five ␣v integrins, although causing lethality, FN-null embryos fail to form notochord or somites allows considerable development and organogenesis (George et al., 1993; Georges-Labouesse et al., 1996), including, most notably, extensive vasculogenesis and whereas embryos null for either (Yang et al., 1993, 1995) angiogenesis. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Secretion of Placental Growth Factor Promotes Angiogenesis by Enhanced Fibroblast-Like Synoviocytes Via Cadherin-11 Interaction
Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor This information is current as of September 23, 2021. Su-Jung Park, Ki-Jo Kim, Wan-Uk Kim and Chul-Soo Cho J Immunol 2014; 192:3003-3010; Prepublished online 26 February 2014; doi: 10.4049/jimmunol.1302177 http://www.jimmunol.org/content/192/7/3003 Downloaded from References This article cites 50 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/192/7/3003.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 23, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts Errata An erratum has been published regarding this article. Please see next page or: /content/192/10/4932.full.pdf The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor Su-Jung Park,* Ki-Jo Kim,† Wan-Uk Kim,*,† and Chul-Soo Cho*,‡ Bone marrow–derived mesenchymal stem cells (MSC) exist in the synovium of patients with rheumatoid arthritis (RA), yet the role of MSC in RA is elusive. -
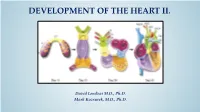
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Abnormalities of Placental Development and Function Are
bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Weston Troja1, Kathryn J. Owens1, Jennifer Courtney1, Andrea C. Hinton3, Robert B. Hinton3, James F. Cnota2 and Helen N. Jones1* 1. Center for Fetal and Placental Therapy, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 2. Heart Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 3. TriHealth Maternal Fetal Medicine, 375 Dixsmyth Ave, Cincinnati Ohio *Corresponding Author: Helen N. Jones [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Background: Birthweight is a critical predictor of congenital heart disease (CHD) surgical outcomes. Hypoplastic left heart syndrome (HLHS) is cyanotic CHD with known fetal growth restriction and placental abnormalities. Transposition of the great arteries (TGA) is cyanotic CHD with normal fetal growth. Comparison of the placenta in these diagnoses may provide insights on the fetal growth abnormality of CHD. Methods: Clinical data and placental histology from placentas associated with Transposition of the Great Arteries (TGA) were analyzed for gross pathology, morphology, maturity and vascularity and compared to both control and previously analyzed HLHS placentas [1]. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

COMMENTARY the First Evidence of the Tumor-Induced Angiogenesis in Vivo by Using the Chorioallantoic Membrane Assay Dated 1913
Leukemia (2004) 18, 1350–1351 & 2004 Nature Publishing Group All rights reserved 0887-6924/04 $30.00 www.nature.com/leu COMMENTARY The first evidence of the tumor-induced angiogenesis in vivo by using the chorioallantoic membrane assay dated 1913 Domenico Ribatti1 1Department of Human Anatomy and Histology, University of Bari Medical School, Bari, Italy Leukemia (2004) 18, 1350–1351. doi:10.1038/sj.leu.2403411 tional characterization of the immune system in the chick Published online 17 June 2004 embryo. Early lymphoid cells deriving from the yolk sac and spleen are usually recognizable in the thymus on day 8 and in Virchow, the founder of pathological anatomy, drew attention to the bursa of Fabricius on day 11.6 Thymus cells are present by the huge number of blood vessels in a tumor mass as long ago as day 11 and cell-mediated immunity has been demonstrated by 1865. Tumor vascularization was first studied systematically by day 13–14.7 The chick embryo and the nude mouse are 1 Goldman, who described the vasoproliferative response of the immunological incompetent hosts and do not reject tissues organ in which a tumor develops as follows: ‘The normal blood from a foreign source. Indeed, the chick embryo cannot mount vessels of the organs in which the tumor is developing are an ‘immune’ response to foreign tumor cells until well after day disturbed by chaotic growth, there is a dilatation and spiralling 12, but it can respond to tumor cells by infiltration of monocytes of the affected vessels, marked capillary budding and new vessel and inflammatory-like cells such as avian heterophils. -

Angiogenesis: an Alternate Approach to Cardiovascular Disease Treatment
University of Southern Maine USM Digital Commons Thinking Matters Symposium Archive Student Scholarship Spring 2017 Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment Scott Ouillette Southern Maine Community College Follow this and additional works at: https://digitalcommons.usm.maine.edu/thinking_matters Part of the Alternative and Complementary Medicine Commons, and the Cardiovascular Diseases Commons Recommended Citation Ouillette, Scott, "Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment" (2017). Thinking Matters Symposium Archive. 99. https://digitalcommons.usm.maine.edu/thinking_matters/99 This Poster Session is brought to you for free and open access by the Student Scholarship at USM Digital Commons. It has been accepted for inclusion in Thinking Matters Symposium Archive by an authorized administrator of USM Digital Commons. For more information, please contact [email protected]. Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment Scott Ouillette, Southern Maine Community College. Instructor: Lisa Dietrich, Southern Maine Community College/Maine Medical Center Abstract Methods As cardiovascular disease continues to be the number one killer of The way angiogenesis is triggered in the body has many different steps and can be humans across the globe, a new approach in treatment and prevention broken down into 3 categories: The mechanical trigger, the chemical trigger, and needs to be found. Angiogenesis is a promising young field of study that the molecular trigger. The process as a whole is more commonly known as the can change the way we treat patients with cardiovascular angiogenesis signaling cascade. complications. Because mechanical triggers are not well known and chemical triggers is the body's natural response to blockages during tissue hypoxia, the molecular trigger makes more sense for growth factor therapy. -

Conditional Mutation of Hand1 in the Mouse Placenta Disrupts Placental Vascular Development Resulting in Fetal Loss in Both Early and Late Pregnancy
International Journal of Molecular Sciences Article Conditional Mutation of Hand1 in the Mouse Placenta Disrupts Placental Vascular Development Resulting in Fetal Loss in Both Early and Late Pregnancy Jennifer A. Courtney 1, Rebecca L. Wilson 2,3, James Cnota 4 and Helen N. Jones 2,3,* 1 Center for Fetal and Placental Therapy, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; [email protected] 2 Center for Research in Perinatal Outcomes, College of Medicine, University of Florida, Gainesville, FL 32603, USA; rebecca.wilson@ufl.edu 3 Department of Physiology and Functional Genomics, College of Medicine, University of Florida, Gainesville, FL 32603, USA 4 Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; [email protected] * Correspondence: jonesh@ufl.edu; Tel.: +1-352-846-1503 Abstract: Congenital heart defects (CHD) affect approximately 1% of all live births, and often require complex surgeries at birth. We have previously demonstrated abnormal placental vascularization in human placentas from fetuses diagnosed with CHD. Hand1 has roles in both heart and placen- tal development and is implicated in CHD development. We utilized two conditionally activated Hand1A126fs/+ murine mutant models to investigate the importance of cell-specific Hand1 on placental development in early (Nkx2-5Cre) and late (Cdh5Cre) pregnancy. Embryonic lethality occurred in Citation: Courtney, J.A.; Wilson, R.L.; Nkx2-5Cre/Hand1A126fs/+ embryos with marked fetal demise occurring after E10.5 due to a failure Cnota, J.; Jones, H.N. Conditional in placental labyrinth formation and therefore the inability to switch to hemotrophic nutrition or Mutation of Hand1 in the Mouse Placenta Disrupts Placental Vascular maintain sufficient oxygen transfer to the fetus. -
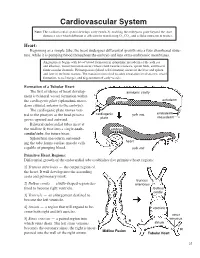
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle.