HGF Upregulation Contributes to Angiogenesis in Mice with Keratinocyte-Specific Smad2 Deletion Kristina E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All V Integrins
Cell, Vol. 95, 507–519, November 13, 1998, Copyright ©1998 by Cell Press Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All ␣v Integrins Bernhard L. Bader,*‡ Helen Rayburn,* their ligands. Significant expression of ␣v integrins has Denise Crowley,* and Richard O. Hynes*† been noted, in particular, in neural crest cells (Delannet * Howard Hughes Medical Institute et al., 1994), glial cells (Hirsch et al., 1994; Milner and Center for Cancer Research ffrench-Constant, 1994), muscle (Hirsch et al., 1994; Mc- and Department of Biology Donald et al., 1995; Martin and Sanes, 1997), osteoclasts Massachusetts Institute of Technology (Va¨ a¨ na¨ nen and Horton, 1995), epithelia (␣v6; Breuss et Cambridge, Massachusetts 02139 al., 1995; Huang et al., 1996), and blood vessels during development (Brooks et al., 1994a; Drake et al., 1995; Friedlander et al., 1995, 1996) or angiogenesis in re- Summary sponse to tumors (Brooks et al., 1994a, 1994b, 1996, 1998; Varner et al., 1995). ␣v integrins have been implicated in many develop- Among the ligands for various ␣v integrins is fibro- mental processes and are therapeutic targets for inhi- nectin (FN), and results on mouse embryos lacking FN bition of angiogenesis and osteoporosis. Surprisingly, or FN receptor integrins suggest that ␣v integrins might ablation of the gene for the ␣v integrin subunit, elimi- be important receptors for FN during early development. nating all five ␣v integrins, although causing lethality, FN-null embryos fail to form notochord or somites allows considerable development and organogenesis (George et al., 1993; Georges-Labouesse et al., 1996), including, most notably, extensive vasculogenesis and whereas embryos null for either (Yang et al., 1993, 1995) angiogenesis. -

Secretion of Placental Growth Factor Promotes Angiogenesis by Enhanced Fibroblast-Like Synoviocytes Via Cadherin-11 Interaction
Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor This information is current as of September 23, 2021. Su-Jung Park, Ki-Jo Kim, Wan-Uk Kim and Chul-Soo Cho J Immunol 2014; 192:3003-3010; Prepublished online 26 February 2014; doi: 10.4049/jimmunol.1302177 http://www.jimmunol.org/content/192/7/3003 Downloaded from References This article cites 50 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/192/7/3003.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 23, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts Errata An erratum has been published regarding this article. Please see next page or: /content/192/10/4932.full.pdf The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Interaction of Mesenchymal Stem Cells with Fibroblast-like Synoviocytes via Cadherin-11 Promotes Angiogenesis by Enhanced Secretion of Placental Growth Factor Su-Jung Park,* Ki-Jo Kim,† Wan-Uk Kim,*,† and Chul-Soo Cho*,‡ Bone marrow–derived mesenchymal stem cells (MSC) exist in the synovium of patients with rheumatoid arthritis (RA), yet the role of MSC in RA is elusive. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

COMMENTARY the First Evidence of the Tumor-Induced Angiogenesis in Vivo by Using the Chorioallantoic Membrane Assay Dated 1913
Leukemia (2004) 18, 1350–1351 & 2004 Nature Publishing Group All rights reserved 0887-6924/04 $30.00 www.nature.com/leu COMMENTARY The first evidence of the tumor-induced angiogenesis in vivo by using the chorioallantoic membrane assay dated 1913 Domenico Ribatti1 1Department of Human Anatomy and Histology, University of Bari Medical School, Bari, Italy Leukemia (2004) 18, 1350–1351. doi:10.1038/sj.leu.2403411 tional characterization of the immune system in the chick Published online 17 June 2004 embryo. Early lymphoid cells deriving from the yolk sac and spleen are usually recognizable in the thymus on day 8 and in Virchow, the founder of pathological anatomy, drew attention to the bursa of Fabricius on day 11.6 Thymus cells are present by the huge number of blood vessels in a tumor mass as long ago as day 11 and cell-mediated immunity has been demonstrated by 1865. Tumor vascularization was first studied systematically by day 13–14.7 The chick embryo and the nude mouse are 1 Goldman, who described the vasoproliferative response of the immunological incompetent hosts and do not reject tissues organ in which a tumor develops as follows: ‘The normal blood from a foreign source. Indeed, the chick embryo cannot mount vessels of the organs in which the tumor is developing are an ‘immune’ response to foreign tumor cells until well after day disturbed by chaotic growth, there is a dilatation and spiralling 12, but it can respond to tumor cells by infiltration of monocytes of the affected vessels, marked capillary budding and new vessel and inflammatory-like cells such as avian heterophils. -

Angiogenesis: an Alternate Approach to Cardiovascular Disease Treatment
University of Southern Maine USM Digital Commons Thinking Matters Symposium Archive Student Scholarship Spring 2017 Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment Scott Ouillette Southern Maine Community College Follow this and additional works at: https://digitalcommons.usm.maine.edu/thinking_matters Part of the Alternative and Complementary Medicine Commons, and the Cardiovascular Diseases Commons Recommended Citation Ouillette, Scott, "Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment" (2017). Thinking Matters Symposium Archive. 99. https://digitalcommons.usm.maine.edu/thinking_matters/99 This Poster Session is brought to you for free and open access by the Student Scholarship at USM Digital Commons. It has been accepted for inclusion in Thinking Matters Symposium Archive by an authorized administrator of USM Digital Commons. For more information, please contact [email protected]. Angiogenesis: An Alternate Approach To Cardiovascular Disease Treatment Scott Ouillette, Southern Maine Community College. Instructor: Lisa Dietrich, Southern Maine Community College/Maine Medical Center Abstract Methods As cardiovascular disease continues to be the number one killer of The way angiogenesis is triggered in the body has many different steps and can be humans across the globe, a new approach in treatment and prevention broken down into 3 categories: The mechanical trigger, the chemical trigger, and needs to be found. Angiogenesis is a promising young field of study that the molecular trigger. The process as a whole is more commonly known as the can change the way we treat patients with cardiovascular angiogenesis signaling cascade. complications. Because mechanical triggers are not well known and chemical triggers is the body's natural response to blockages during tissue hypoxia, the molecular trigger makes more sense for growth factor therapy. -
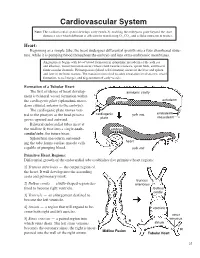
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Identification of Novel Hemangioblast Genes in the Early Chick Embryo
cells Communication Identification of Novel Hemangioblast Genes in the Early Chick Embryo José Serrado Marques 1, Vera Teixeira 2 ID , António Jacinto 1 and Ana Teresa Tavares 1,* ID 1 CEDOC, Chronic Diseases Research Centre, NOVA Medical School, Universidade NOVA de Lisboa, Campo dos Mártires da Pátria, 130, 1169-056 Lisbon, Portugal; [email protected] (J.S.M.); [email protected] (A.J.) 2 Instituto Gulbenkian de Ciência, Rua da Quinta Grande, 6, 2780-156 Oeiras, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-218-803-101 Received: 10 December 2017; Accepted: 27 January 2018; Published: 31 January 2018 Abstract: During early vertebrate embryogenesis, both hematopoietic and endothelial lineages derive from a common progenitor known as the hemangioblast. Hemangioblasts derive from mesodermal cells that migrate from the posterior primitive streak into the extraembryonic yolk sac. In addition to primitive hematopoietic cells, recent evidence revealed that yolk sac hemangioblasts also give rise to tissue-resident macrophages and to definitive hematopoietic stem/progenitor cells. In our previous work, we used a novel hemangioblast-specific reporter to isolate the population of chick yolk sac hemangioblasts and characterize its gene expression profile using microarrays. Here we report the microarray profile analysis and the identification of upregulated genes not yet described in hemangioblasts. These include the solute carrier transporters SLC15A1 and SCL32A1, the cytoskeletal protein RhoGap6, the serine protease CTSG, the transmembrane receptor MRC1, the transcription factors LHX8, CITED4 and PITX1, and the previously uncharacterized gene DIA1R. Expression analysis by in situ hybridization showed that chick DIA1R is expressed not only in yolk sac hemangioblasts but also in particular intraembryonic populations of hemogenic endothelial cells, suggesting a potential role in the hemangioblast-derived hemogenic lineage. -

C-Met As a Key Factor Responsible for Sustaining Undifferentiated Phenotype and Therapy Resistance in Renal Carcinomas
cells Review C-Met as a Key Factor Responsible for Sustaining Undifferentiated Phenotype and Therapy Resistance in Renal Carcinomas Paulina Marona 1 , Judyta Górka 1 , Jerzy Kotlinowski 1 , Marcin Majka 2 , Jolanta Jura 1 and Katarzyna Miekus 1,* 1 Department of General Biochemistry, Faculty of Biochemistry, Biopphisics and Biotechnology, Jagiellonian University, Gronostajowa Street 7, 30-387 Krakow, Poland; [email protected] (P.M.); [email protected] (J.G.); [email protected] (J.K.); [email protected] (J.J.) 2 Department of Transplantation, Jagiellonian University Medical College, Jagiellonian University, Wielicka 265, 30-663 Krakow, Poland; [email protected] * Correspondence: [email protected] Received: 28 February 2019; Accepted: 19 March 2019; Published: 22 March 2019 Abstract: C-Met tyrosine kinase receptor plays an important role under normal and pathological conditions. In tumor cells’ overexpression or incorrect activation of c-Met, this leads to stimulation of proliferation, survival and increase of motile activity. This receptor is also described as a marker of cancer initiating cells. The latest research shows that the c-Met receptor has an influence on the development of resistance to targeted cancer treatment. High c-Met expression and activation in renal cell carcinomas is associated with the progression of the disease and poor survival of patients. C-Met receptor has become a therapeutic target in kidney cancer. However, the therapies used so far using c-Met tyrosine kinase inhibitors demonstrate resistance to treatment. On the other hand, the c-Met pathway may act as an alternative target pathway in tumors that are resistant to other therapies. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography -

Lymphangiogenesis, Inflammation and Metastasis
ANTICANCER RESEARCH 25: 4503-4512 (2005) Review Lymphangiogenesis, Inflammation and Metastasis SEBASTIAN F. SCHOPPMANN Department of Surgery, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria Abstract. The lymphatic vascular system is necessary for the cells (1). In the periphery, antigen-presenting cells and return of extravasated interstitial fluid and macromolecules to lymphocytes enter the capillaries and migrate through the the blood circulation, for immune defense, and for the uptake lymphatic system to the lymph nodes to elicit acquired of dietary fats. Impaired functioning of lymphatic vessels results immune response in the body. In the small intestine, the in lymphedema, whereas tumor-associated lymphangiogenesis lymphatics play a special role in the process of fat may contribute to the spread of cancer cells from solid tumors. absorption. Recent studies have identified lymphatic molecular markers and This extensive drainage network is lined by a single, thin, growth factors necessary for lymphangiogenesis. In particular, non-fenestrated lymphatic endothelial cell (LECs) layer (2). lymphatic endothelial receptor tyrosine kinase VEGFR-3, and An incomplete basement membrane is characteristic, and the its ligands VEGF-C and VEGF-D, are major players in lymphatic endothelial cells are anchored to the extracellular promoting lymphatic vascular growth both during development matrix through elastic fibers, which keep the vessels open, and in pathological conditions. Lymphatic vessels play a crucial allowing for changes in interstitial pressure (3). role in a variety of human cancers, since invasion of lymphatic Two theories about the development of the lymphatic vessels by tumor cells and subsequent development of lymph system were proposed at the beginning of the last century: i) node metastases significantly influence the prognosis of cancer the venous origin of lymphatic vessels and ii) the de novo patients and, therefore, represent an integral part of tumor formation of primary lymph sacs in the mesenchyme (4, 5).