Downloaded Through Cbioportal to Analyze the Correlation Between Cnas and Expression Levels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer and Development
International Journal of Molecular Sciences Review Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer and Development Charles Ducker * and Peter E. Shaw * Queen’s Medical Centre, School of Life Sciences, University of Nottingham, Nottingham NG7 2UH, UK * Correspondence: [email protected] (C.D.); [email protected] (P.E.S.) Abstract: Genome expansion, whole genome and gene duplication events during metazoan evolution produced an extensive family of ETS genes whose members express transcription factors with a conserved winged helix-turn-helix DNA-binding domain. Unravelling their biological roles has proved challenging with functional redundancy manifest in overlapping expression patterns, a common consensus DNA-binding motif and responsiveness to mitogen-activated protein kinase signalling. Key determinants of the cellular repertoire of ETS proteins are their stability and turnover, controlled largely by the actions of selective E3 ubiquitin ligases and deubiquitinases. Here we discuss the known relationships between ETS proteins and enzymes that determine their ubiquitin status, their integration with other developmental signal transduction pathways and how suppression of ETS protein ubiquitination contributes to the malignant cell phenotype in multiple cancers. Keywords: E3 ligase complex; deubiquitinase; gene fusions; mitogens; phosphorylation; DNA damage 1. Introduction Citation: Ducker, C.; Shaw, P.E. Cell growth, proliferation and differentiation are complex, concerted processes that Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer rely on careful regulation of gene expression. Control over gene expression is maintained and Development. Int. J. Mol. Sci. through signalling pathways that respond to external cellular stimuli, such as growth 2021, 22, 5119. https://doi.org/ factors, cytokines and chemokines, that invoke expression profiles commensurate with 10.3390/ijms22105119 diverse cellular outcomes. -
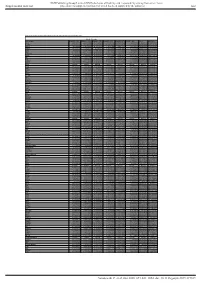
Supl Table 1 for Pdf.Xlsx
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Table S1. Proteomic analysis of liver tissues from 4-months old control and LPTENKO mice. Soluble Fraction Gene names LOG2FC CTL1 LOG2FC CTL2 LOG2FC CTL3 LOG2FC KO1 LOG2FC KO2 LOG2FC KO3 t-test adj Pvalue Aass 0.081686519 -0.097912098 0.016225579 -1.135271836 -0.860535624 -1.263037541 0.0011157 1.206072068 Acsm5 -0.125220746 0.071090866 0.054129881 -0.780692107 -0.882155692 -1.158378647 0.00189031 1.021713016 Gstm2 -0.12707966 0.572554941 -0.445475281 1.952813994 1.856518122 1.561376671 0.00517664 1.865316016 Gstm1 -0.029147714 0.298593425 -0.26944571 0.983159098 0.872028945 0.786125509 0.00721356 1.949464926 Fasn 0.08403202 -0.214149498 0.130117478 1.052480559 0.779734519 1.229308218 0.00383637 0.829422353 Upb1 -0.112438784 -0.137014769 0.249453553 -1.297732445 -1.181999331 -1.495303666 0.00102373 0.184442034 Selenbp2 0.266508271 0.084791964 -0.351300235 -2.040647809 -2.608780781 -2.039865739 0.00107121 0.165425127 Thrsp -0.15001075 0.177999342 -0.027988592 1.54283307 1.603048661 0.927443822 0.00453549 0.612858263 Hyi 0.142635733 -0.183013091 0.040377359 1.325929636 1.119934412 1.181313897 0.00044587 0.053553518 Eci1 -0.119041811 -0.014846366 0.133888177 -0.599970385 -0.555547972 -1.191875541 0.02292305 2.477981171 Aldh1a7 -0.095682449 -0.017781922 0.113464372 0.653424862 0.931724091 1.110750381 0.00356922 0.350756574 Pebp1 -0.06292058 -

Inhibition of Mitochondrial Complex II in Neuronal Cells Triggers Unique
www.nature.com/scientificreports OPEN Inhibition of mitochondrial complex II in neuronal cells triggers unique pathways culminating in autophagy with implications for neurodegeneration Sathyanarayanan Ranganayaki1, Neema Jamshidi2, Mohamad Aiyaz3, Santhosh‑Kumar Rashmi4, Narayanappa Gayathri4, Pulleri Kandi Harsha5, Balasundaram Padmanabhan6 & Muchukunte Mukunda Srinivas Bharath7* Mitochondrial dysfunction and neurodegeneration underlie movement disorders such as Parkinson’s disease, Huntington’s disease and Manganism among others. As a corollary, inhibition of mitochondrial complex I (CI) and complex II (CII) by toxins 1‑methyl‑4‑phenylpyridinium (MPP+) and 3‑nitropropionic acid (3‑NPA) respectively, induced degenerative changes noted in such neurodegenerative diseases. We aimed to unravel the down‑stream pathways associated with CII inhibition and compared with CI inhibition and the Manganese (Mn) neurotoxicity. Genome‑wide transcriptomics of N27 neuronal cells exposed to 3‑NPA, compared with MPP+ and Mn revealed varied transcriptomic profle. Along with mitochondrial and synaptic pathways, Autophagy was the predominant pathway diferentially regulated in the 3‑NPA model with implications for neuronal survival. This pathway was unique to 3‑NPA, as substantiated by in silico modelling of the three toxins. Morphological and biochemical validation of autophagy markers in the cell model of 3‑NPA revealed incomplete autophagy mediated by mechanistic Target of Rapamycin Complex 2 (mTORC2) pathway. Interestingly, Brain Derived Neurotrophic Factor -

X-Linked Diseases: Susceptible Females
REVIEW ARTICLE X-linked diseases: susceptible females Barbara R. Migeon, MD 1 The role of X-inactivation is often ignored as a prime cause of sex data include reasons why women are often protected from the differences in disease. Yet, the way males and females express their deleterious variants carried on their X chromosome, and the factors X-linked genes has a major role in the dissimilar phenotypes that that render women susceptible in some instances. underlie many rare and common disorders, such as intellectual deficiency, epilepsy, congenital abnormalities, and diseases of the Genetics in Medicine (2020) 22:1156–1174; https://doi.org/10.1038/s41436- heart, blood, skin, muscle, and bones. Summarized here are many 020-0779-4 examples of the different presentations in males and females. Other INTRODUCTION SEX DIFFERENCES ARE DUE TO X-INACTIVATION Sex differences in human disease are usually attributed to The sex differences in the effect of X-linked pathologic variants sex specific life experiences, and sex hormones that is due to our method of X chromosome dosage compensation, influence the function of susceptible genes throughout the called X-inactivation;9 humans and most placental mammals – genome.1 5 Such factors do account for some dissimilarities. compensate for the sex difference in number of X chromosomes However, a major cause of sex-determined expression of (that is, XX females versus XY males) by transcribing only one disease has to do with differences in how males and females of the two female X chromosomes. X-inactivation silences all X transcribe their gene-rich human X chromosomes, which is chromosomes but one; therefore, both males and females have a often underappreciated as a cause of sex differences in single active X.10,11 disease.6 Males are the usual ones affected by X-linked For 46 XY males, that X is the only one they have; it always pathogenic variants.6 Females are biologically superior; a comes from their mother, as fathers contribute their Y female usually has no disease, or much less severe disease chromosome. -

USP9X Regulates Centrosome Duplication and Promotes Breast Carcinogenesis
ARTICLE Received 21 Feb 2016 | Accepted 31 Jan 2017 | Published 31 Mar 2017 DOI: 10.1038/ncomms14866 OPEN USP9X regulates centrosome duplication and promotes breast carcinogenesis Xin Li1,*, Nan Song1,*, Ling Liu1, Xinhua Liu1, Xiang Ding2, Xin Song3, Shangda Yang1, Lin Shan1, Xing Zhou1, Dongxue Su1, Yue Wang1, Qi Zhang1, Cheng Cao1, Shuai Ma1,NaYu1, Fuquan Yang2, Yan Wang1, Zhi Yao4, Yongfeng Shang1,5 & Lei Shi1,4 Defective centrosome duplication is implicated in microcephaly and primordial dwarfism as well as various ciliopathies and cancers. Yet, how the centrosome biogenesis is regulated remains poorly understood. Here we report that the X-linked deubiquitinase USP9X is physically associated with centriolar satellite protein CEP131, thereby stabilizing CEP131 through its deubiquitinase activity. We demonstrate that USP9X is an integral component of centrosome and is required for centrosome biogenesis. Loss-of-function of USP9X impairs centrosome duplication and gain-of-function of USP9X promotes centrosome amplification and chromosome instability. Significantly, USP9X is overexpressed in breast carcinomas, and its level of expression is correlated with that of CEP131 and higher histologic grades of breast cancer. Indeed, USP9X, through regulation of CEP131 abundance, promotes breast carcino- genesis. Our experiments identify USP9X as an important regulator of centrosome biogenesis and uncover a critical role for USP9X/CEP131 in breast carcinogenesis, supporting the pursuit of USP9X/CEP131 as potential targets for breast cancer intervention. 1 2011 Collaborative Innovation Center of Tianjin for Medical Epigenetics, Tianjin Key Laboratory of Medical Epigenetics, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China. 2 Laboratory of Proteomics, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China. -

The Roles of Trim15 and UCHL3 in the Ubiquitin-Mediated Cell Cycle Regulation Katerina Jerabkova
The roles of Trim15 and UCHL3 in the ubiquitin-mediated cell cycle regulation Katerina Jerabkova To cite this version: Katerina Jerabkova. The roles of Trim15 and UCHL3 in the ubiquitin-mediated cell cycle regulation. Cellular Biology. Université de Strasbourg; Univerzita Karlova (Prague), 2019. English. NNT : 2019STRAJ034. tel-02884583 HAL Id: tel-02884583 https://tel.archives-ouvertes.fr/tel-02884583 Submitted on 30 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITÉ DE STRASBOURG ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTÉ IGBMC - CNRS UMR 7104 - Inserm U 1258 THÈSE présentée par : Kateřina JEŘÁBKOVÁ soutenue le : 09 Octobre 2019 pour obtenir le grade de : Docteur de l’université de Strasbourg Discipline/ Spécialité : Aspects moléculaires et cellulaires de la biologie Les rôles de Trim15 et UCHL3 dans la régulation, médiée par l’ubiquitine, du cycle cellulaire. The roles of Trim15 and UCHL3 in the ubiquitin-mediated cell cycle regulation. THÈSE dirigée par : Mme SUMARA Izabela PhD, Université de Strasbourg Mme CHAWENGSAKSOPHAK -

SUPPORTING INFORMATION Proteomic Evaluation of the Acute
SUPPORTING INFORMATION Proteomic evaluation of the acute radiation syndrome of the gastrointestinal tract in a murine total-body irradiation model. Weiliang Huang*, Jianshi Yu*, Jace W. Jones*, Claire L. Carter*, Keely Pierzchalski*, Gregory Tudor†, Catherine Booth†, Thomas J. MacVittie‡, Maureen A. Kane* §. *University of Maryland, School of Pharmacy, Department of Pharmaceutical Sciences, Baltimore, MD; †Epistem Ltd, Manchester, UK; ‡ University of Maryland, School of Medicine, Department of Radiation Oncology, Baltimore, MD §Correspondence: Maureen A. Kane University of Maryland, School of Pharmacy Department of Pharmaceutical Sciences 20 N. Pine Street, Room 723 Baltimore, MD 21201 Phone: (410) 706-5097 Fax: (410) 706-0886 Email: [email protected] Retinoid analysis. Retinoid levels were determined by liquid chromatography-multistage tandem mass spectrometry (LC-MRM3) which is an LC-MS/MS method utilizing two distinct fragmentation events for enhanced selectivity (Jones et al. 2015). Preparation of mouse intestinal tissue included flushing out contents with PBS. The jejunum and ileum were isolated from the small intestine and snap frozen with liquid nitrogen and stored at -80 °C until extraction. Tissues were homogenized in saline and extraction of retinoids was performed under yellow lights using a two-step liquid-liquid extraction that has been described in detail previously using 4,4-dimethyl- RA as an internal standard (Kane et al. 2005; Kane et al. 2008b; Kane and Napoli 2010; Jones et al. 2015). Levels of RA were measured using a Shimadzu Prominence UFLC XR liquid chromatography system (Shimadzu, Columbia, MD) coupled to an AB Sciex 5500 QTRAP hybrid triple quadrupole mass spectrometer (AB Sciex, Framingham, MA) using atmospheric pressure chemical ionization (APCI) operated in positive ion mode as previously described (Jones et al. -

Biochemical Investigation of the Ubiquitin Carboxyl-Terminal Hydrolase Family" (2015)
Purdue University Purdue e-Pubs Open Access Dissertations Theses and Dissertations Spring 2015 Biochemical investigation of the ubiquitin carboxyl- terminal hydrolase family Joseph Rashon Chaney Purdue University Follow this and additional works at: https://docs.lib.purdue.edu/open_access_dissertations Part of the Biochemistry Commons, Biophysics Commons, and the Molecular Biology Commons Recommended Citation Chaney, Joseph Rashon, "Biochemical investigation of the ubiquitin carboxyl-terminal hydrolase family" (2015). Open Access Dissertations. 430. https://docs.lib.purdue.edu/open_access_dissertations/430 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. *UDGXDWH6FKRRO)RUP 8SGDWHG PURDUE UNIVERSITY GRADUATE SCHOOL Thesis/Dissertation Acceptance 7KLVLVWRFHUWLI\WKDWWKHWKHVLVGLVVHUWDWLRQSUHSDUHG %\ Joseph Rashon Chaney (QWLWOHG BIOCHEMICAL INVESTIGATION OF THE UBIQUITIN CARBOXYL-TERMINAL HYDROLASE FAMILY Doctor of Philosophy )RUWKHGHJUHHRI ,VDSSURYHGE\WKHILQDOH[DPLQLQJFRPPLWWHH Chittaranjan Das Angeline Lyon Christine A. Hrycyna George M. Bodner To the best of my knowledge and as understood by the student in the Thesis/Dissertation Agreement, Publication Delay, and Certification/Disclaimer (Graduate School Form 32), this thesis/dissertation adheres to the provisions of Purdue University’s “Policy on Integrity in Research” and the use of copyrighted material. Chittaranjan Das $SSURYHGE\0DMRU3URIHVVRU V BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB $SSURYHGE\R. E. Wild 04/24/2015 +HDGRIWKH'HSDUWPHQW*UDGXDWH3URJUDP 'DWH BIOCHEMICAL INVESTIGATION OF THE UBIQUITIN CARBOXYL-TERMINAL HYDROLASE FAMILY Dissertation Submitted to the Faculty of Purdue University by Joseph Rashon Chaney In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2015 Purdue University West Lafayette, Indiana ii All of this I dedicate wife, Millicent, to my faithful and beautiful children, Josh and Caleb. -

Protein Symbol Protein Name Rank Metric Score 4F2 4F2 Cell-Surface
Supplementary Table 2 Supplementary Table 2. Ranked list of proteins present in anti-Sema4D treated macrophage conditioned media obtained in the GSEA analysis of the proteomic data. Proteins are listed according to their rank metric score, which is the score used to position the gene in the ranked list of genes of the GSEA. Values are obtained from comparing Sema4D treated RAW conditioned media versus REST, which includes untreated, IgG treated and anti-Sema4D added RAW conditioned media. GSEA analysis was performed under standard conditions in November 2015. Protein Rank metric symbol Protein name score 4F2 4F2 cell-surface antigen heavy chain 2.5000 PLOD3 Procollagen-lysine,2-oxoglutarate 5-dioxygenase 3 1.4815 ELOB Transcription elongation factor B polypeptide 2 1.4350 ARPC5 Actin-related protein 2/3 complex subunit 5 1.2603 OSTF1 teoclast-stimulating factor 1 1.2500 RL5 60S ribomal protein L5 1.2135 SYK Lysine--tRNA ligase 1.2135 RL10A 60S ribomal protein L10a 1.2135 TXNL1 Thioredoxin-like protein 1 1.1716 LIS1 Platelet-activating factor acetylhydrolase IB subunit alpha 1.1067 A4 Amyloid beta A4 protein 1.0911 H2B1M Histone H2B type 1-M 1.0514 UB2V2 Ubiquitin-conjugating enzyme E2 variant 2 1.0381 PDCD5 Programmed cell death protein 5 1.0373 UCHL3 Ubiquitin carboxyl-terminal hydrolase isozyme L3 1.0061 PLEC Plectin 1.0061 ITPA Inine triphphate pyrophphatase 0.9524 IF5A1 Eukaryotic translation initiation factor 5A-1 0.9314 ARP2 Actin-related protein 2 0.8618 HNRPL Heterogeneous nuclear ribonucleoprotein L 0.8576 DNJA3 DnaJ homolog subfamily -

USP9X Monoclonal Antibody (M05A), Clone 5D7
USP9X monoclonal antibody (M05A), clone 5D7 Catalog # : H00008239-M05A 規格 : [ 200 uL ] List All Specification Application Image Product Mouse monoclonal antibody raised against a partial recombinant Western Blot (Tissue lysate) Description: USP9X. Immunogen: USP9X (NP_068706, 1 a.a. ~ 90 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Sequence: MTATTRGSPVGGNDNQGQAPDGQSQPPLQQNQTSSPDSSNENSPATP PDEQGQGDAPPQLEDEEPAFPHTDLAKLDDMINRPRWVVPVLP enlarge Western Blot (Cell lysate) Host: Mouse Reactivity: Human Isotype: IgG1 Kappa Quality Control Antibody Reactive Against Recombinant Protein. enlarge Testing: Western Blot (Recombinant protein) ELISA Western Blot detection against Immunogen (35.64 KDa) . Storage Buffer: In ascites fluid Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Datasheet: Download Applications Western Blot (Tissue lysate) Page 1 of 3 2020/6/25 USP9X monoclonal antibody (M05A), clone 5D7. Western Blot analysis of USP9X expression in human placenta. Protocol Download Western Blot (Cell lysate) USP9X monoclonal antibody (M05A), clone 5D7. Western Blot analysis of USP9X expression in Jurkat(Cat # L017V1 ). Protocol Download Western Blot (Recombinant protein) Protocol Download ELISA Gene Information Entrez GeneID: 8239 GeneBank NM_021906 Accession#: Protein NP_068706 Accession#: Gene Name: USP9X Gene Alias: DFFRX,FAF,FAM Gene ubiquitin specific peptidase 9, X-linked Description: Omim ID: 300072 Gene Ontology: Hyperlink Gene Summary: This gene is a member of the peptidase C19 family and encodes a protein that is similar to ubiquitin-specific proteases. Though this gene is located on the X chromosome, it escapes X-inactivation. Mutations in this gene have been associated with Turner syndrome. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. -

Differentially Expressed Full-Length, Fusion and Novel Isoforms Transcripts-Based Signature of Well-Differentiated Keratinized Oral Squamous Cell Carcinoma
www.oncotarget.com Oncotarget, 2020, Vol. 11, (No. 34), pp: 3227-3243 Research Paper Differentially expressed full-length, fusion and novel isoforms transcripts-based signature of well-differentiated keratinized oral squamous cell carcinoma Neetu Singh1,*, Dinesh Kumar Sahu1,*, Ratnesh Kumar Tripathi1,*, Archana Mishra1,2, Hari Shyam1, Pratap Shankar1, Mayank Jain1, Nawazish Alam1, Anil Kumar1, Abhishek Mishra1, Rebecca Chowdhry3, Anjana Singh4, Sameer Gupta5, Divya Mehrotra6, Preeti Agarwal7, Madhu Mati Goel7, Arun Chaturvedi5, Satya Prakash Agarwal8, Manish Bajpai9, Devendra Kumar Gupta10, Madan Lal Brahma Bhatt11 and Ravi Kant12 1Department of Molecular Biology, Center for Advance Research, King George's Medical University, Lucknow, India 2Department of Surgery, King George’s Medical University, Lucknow, India 3Department of Periodontology, All India Institute of Medical Sciences, Rishikesh, India 4Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh, India 5Department of Surgical Oncology, King George's Medical University, Lucknow, India 6Department of Oral and Maxillofacial Surgery, King George's Medical University, Lucknow, India 7Department of Pathology, King George's Medical University, Lucknow, India 8Department of Otorhinolaryngology, King George's Medical University, Lucknow, India 9Department of Physiology, King George's Medical University, Lucknow, India 10Department of Pediatric Surgery, Super Speciality Pediatric Hospital and Post Graduate Teaching Institute, Noida, India 11Department of Radiotherapy, King George's Medical University, Lucknow, India 12Department of Surgical Oncology, All India Institute of Medical Sciences, Rishikesh, India *These authors contributed equally to this work Correspondence to: Neetu Singh, email: [email protected] Keywords: oral tongue squamous cell carcinoma; microarray; transcriptomics; integrative bioinformatics; differentially expressed gene Received: May 11, 2020 Accepted: July 14, 2020 Published: August 25, 2020 Copyright: Singh et al.