View Tive Response
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
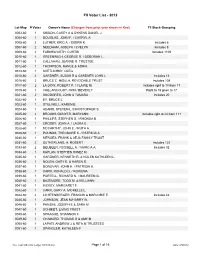
FII Voter Ledger
FII Voter List - 2012 Lot-Map # Votes Owner's Name (Changes from prior year shown in Red) FII Stock Grouping 0003-60 1 SISSON, CAREY A & DYKENS DANIEL J 0004-60 1 DOUGLAS, JOHN F. / CHERYL A. 0005-60 2 LUTHER, ERIC A. / DEBRA K. Includes 6 0007-60 2 NEEDHAM, JOSEPH / EVELYN Includes 8 0009-60 2 FARNSWORTH, CURTIS Includes 1109 0010-60 1 GREENWICH, GEORGE R. / DEBORAH L. 0011-60 1 CALLAHAN, JOANNE R. TRUSTEE 0012-60 1 THOMPSON, RANCE & ADRIA 0013-60 1 SVETLICHNY, OLEG 0015-60 2 GARDNER, SUSAN B & GARDNER JOHN L Includes 14 0016-60 2 BRUCE C. NISULA, REVOCABLE TRUST Includes 108 0017-60 2 LA DOW, ROBERT P. / CLAIRE M. Includes right to 18 from 17 0019-60 1 VAILLANCOURT, RON / BEVERLY Right to 18 given to 17 0021-60 2 SNODGRES, JOHN & TAMARA Includes 20 0022-60 1 EY, BRUCE L. 0023-60 1 STILLWELL, KAREN B. 0024-60 1 ADAMS, STEVEN L./CHRISTOPHER S. 0025-60 2 BROOKS-GONYER, MARYANN Includes right to 24 from 111 0026-60 1 PHILLIPS, STEPHEN S. / RHONDA B. 0027-60 1 CROSBY, JOHN A. / LAURA E. 0028-60 1 MCCARTHY, JOHN E. / RUTH A. 0029-60 1 POUNDS, THEODORE K. / PATRICIA A. 0030-60 1 MERGES, FRANK & AEJA FAMILY TRUST 0031-60 2 SUTHERLAND, H. ROBERT Includes 122 0033-60 2 DEUBLER, RUSSELL A. / MARCIA A. Includes 32 0034-60 1 KAPLAN, STEPHEN H/INEZ M 0035-60 1 GARDNER, KENNETH R. & NOLEN KATHLEEN L. 0036-60 1 NOLEN, GARY E. & KAREN R. 0037-60 1 DONOVAN, JOHN H. -

Metroplus MLTC Provider Directory Table of Contents
MetroPlus MLTC Provider Directory Table of Contents Introduction ..........................................................................................................................3 Adult Day Health Care .......................................................................................................5 AIDS Adult Day Health Care Centers ..............................................................................7 Audiology/Hearing ..............................................................................................................8 Certified Home Health Care ..............................................................................................9 Consumer Directed Personal Care Services ...............................................................11 Durable Medical Equipment (DME) ................................................................................12 Facility Based Occupational Therapy ............................................................................25 Facility Based Physical Therapy .....................................................................................26 Facility Based Speech Therapy ......................................................................................27 Home Delivered Meals / Congregate Meals .................................................................28 Non-Emergent Transportation ........................................................................................30 Orthotics and Prosthetics.................................................................................................34 -

Peripheral Nerve Society I
Peripheral Nerve Society i Officers of the Society Eva L. Feldman, President Douglas W. Zochodne, President-Elect David R. Cornblath, Secretary/Treasurer Amber M. Millen, Executive Secretary Board Members Patricia J. Armati Richard A.C. Hughes Giuseppe Lauria Mary M. Reilly Angelo E. Schenone Michael E. Shy A. Gordon Smith Hugh J. Willison Scientific Program Committee Michael E. Shy, Chair M. Laura Feltri, Co-Chair Wendy M. Campana Angelika F. Hahn Ahmet Höke Jean-Marc Léger Mary M. Reilly James W. Russell Zarife Sahenk J. Robinson Singleton Hugh J. Willison Conference Organizing Committee Local Organizing and Liaison Committees (Universitätsklinikum Würzburg) Klaus V. Toyka, Würzburg, Chair Claudia L. Sommer, Würzburg, Co-Chair Guido Stoll, Würzburg Hans-Peter Hartung, Düsseldorf Rudolf Martini, Würzburg Michael Sendtner, Würzburg CME Joint Sponsorship University of California, San Diego School of Medicine ii The Society The Peripheral Nerve Study Group (PNSG) had its origins in a meeting organized by A. K. Asbury in Carville, Louisiana, in 1974. The success of the meeting led to the formation of the Peripheral Nerve Club, later changing its name to the PNSG, to bring together clinicians and basic scientists interested in peripheral neuropathy and the neurobiology of peripheral nerve. Meetings were held every two years, successively. The Executive Committee consisted of all those who had organized meetings. The PNSG became affiliated to the Research Group on Neuromuscular Diseases (RGND) of the World Federation of Neurology and the members of the Executive Committee of the PNSG were ex- officio members of the Executive Committee of the RGND, responsible for organizing the quadrennial International Congress on Neuromuscular Diseases. -

Official Records
SECTION C SEPTEMBER 25, 2017 BANKER & TRADESMAN Official Records MASSACHUSETTS MARKET STATISTICS INDEX County Sales Charts In This Week’s Issue Number Of Condo Sales None 35003,500 Real Estate Records 28002,800 PAGE COUNTY TRANSACTIONS THRU PAGE COUNTY TRANSACTIONS THRU C2 Suffolk ........ 09/08/17 C11 Franklin ....... 09/08/17 21002,100 C4 Barnstable ..... 09/08/17 C11 Hampden ...... 09/08/17 14001,400 C6 Berkshire Middle 09/08/17 C12 Hampshire ..... 09/08/17 C6 Berkshire North .09/08/17 C13 Middlesex North. 09/08/17 700 700 C6 Berkshire South .09/08/17 C14 Middlesex South. 09/08/17 0 C7 Bristol Fall River 09/08/17 C17 Nantucket ...... 09/08/17 0 Aug. Sept. Oct. Nov. Dec. Jan. Feb. Mar. Apr. May Jun. Jul. Aug. 2010 2010 2011 2011 2011 2011 2011 2011 2011 2011 2011 2011 2011 ’16 ’17 C7 Bristol North ... 09/08/17 C17 Norfolk ........ 09/08/17 Number Change C8 Bristol South ... 09/08/17 C19 Plymouth ...... 09/08/17 3000 Year of Sales Prior Year 3,000 C8 Dukes ......... 09/08/17 C21 Worcester ...... 09/08/17 2400 Aug. 2012 2,481 42.34% 2,400 C9 Essex North .... 09/08/17 C23 Worcester North. 09/08/17 Aug. 2013 2,480 -0.04% 1,8001800 C9 Essex South .... 09/08/17 Aug. 2014 2,226 -10.24% 1,2001200 Aug. 2015 2,434 9.34% Credit Records 600600 Aug. 2016 2,794 14.79% C24 Voluntary Bankruptcies C25 Attachments 00 Aug. 2017 2,643 -5.40% 2007Aug. 2008Aug. 2009Aug. 2010Aug. -
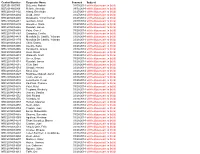
Control Number Requester Name Scanned Subject BLR2014000005 Di Luccio, Patrick 04/09/2014 Withheld Pursuant to (B)(6) BLR2014000
Control Number Requester Name Scanned Subject BLR2014000005 Di Luccio, Patrick 04/09/2014 withheld pursuant to (b)(6) BLR2014000006 Nelson, Amanda 04/15/2014 withheld pursuant to (b)(6) NRC2014034402 Allaraj, Enkelejda 03/27/2014 withheld pursuant to (b)(6) NRC2014034404 Lloyd, Jesse 03/27/2014 withheld pursuant to (b)(6) NRC2014034408 Duraisamy, Vinoth Kumar 03/27/2014 withheld pursuant to (b)(6) NRC2014034412 Gunnam, Kiran 03/27/2014 withheld pursuant to (b)(6) NRC2014034430 Gonzalez, Maria 03/27/2014 withheld pursuant to (b)(6) NRC2014034446 Rudolph, James 03/27/2014 withheld pursuant to (b)(6) NRC2014034458 Potra, Cornel 03/28/2014 withheld pursuant to (b)(6) NRC2014034463 Gonzalez, Cecilia 03/28/2014 withheld pursuant to (b)(6) NRC2014034479 Mendiola De Castillo, Yolanda 03/28/2014 withheld pursuant to (b)(6) NRC2014034479 Mendiola De Castillo, Yolanda 03/28/2014 withheld pursuant to (b)(6) NRC2014034484 Chen, Danny 03/28/2014 withheld pursuant to (b)(6) NRC2014034505 Avelino, Karla 03/28/2014 withheld pursuant to (b)(6) NRC2014034506 Kurotsuchi, James 03/28/2014 withheld pursuant to (b)(6) NRC2014034509 Ross, David 03/28/2014 withheld pursuant to (b)(6) NRC2014034510 Warmuth, Scott 03/28/2014 withheld pursuant to (b)(6) NRC2014034511 Green, Brian 03/28/2014 withheld pursuant to (b)(6) NRC2014034514 Rudolph, James 03/28/2014 withheld pursuant to (b)(6) NRC2014034515 Klein, Bart 03/28/2014 withheld pursuant to (b)(6) NRC2014034519 Sintauli, Herlina 03/28/2014 withheld pursuant to (b)(6) NRC2014034520 Knox, Lisa 03/28/2014 withheld -

Analysis of the Dystrophin Interactome
Analysis of the dystrophin interactome Dissertation In fulfillment of the requirements for the degree “Doctor rerum naturalium (Dr. rer. nat.)” integrated in the International Graduate School for Myology MyoGrad in the Department for Biology, Chemistry and Pharmacy at the Freie Universität Berlin in Cotutelle Agreement with the Ecole Doctorale 515 “Complexité du Vivant” at the Université Pierre et Marie Curie Paris Submitted by Matthew Thorley born in Scunthorpe, United Kingdom Berlin, 2016 Supervisor: Simone Spuler Second examiner: Sigmar Stricker Date of defense: 7th December 2016 Dedicated to My mother, Joy Thorley My father, David Thorley My sister, Alexandra Thorley My fiancée, Vera Sakhno-Cortesi Acknowledgements First and foremost, I would like to thank my supervisors William Duddy and Stephanie Duguez who gave me this research opportunity. Through their combined knowledge of computational and practical expertise within the field and constant availability for any and all assistance I required, have made the research possible. Their overarching support, approachability and upbeat nature throughout, while granting me freedom have made this year project very enjoyable. The additional guidance and supported offered by Matthias Selbach and his team whenever required along with a constant welcoming invitation within their lab has been greatly appreciated. I thank MyoGrad for the collaboration established between UPMC and Freie University, creating the collaboration within this research project possible, and offering research experience in both the Institute of Myology in Paris and the Max Delbruck Centre in Berlin. Vital to this process have been Gisele Bonne, Heike Pascal, Lidia Dolle and Susanne Wissler who have aided in the often complex processes that I am still not sure I fully understand. -
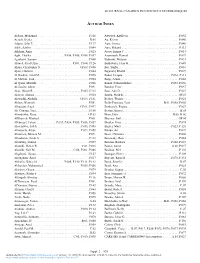
Author Index
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES AUTHOR INDEX Abbass, Mohamad . .P.116 Attwood, Kathleen . P.042 Acsadi, Gyula. B.06 Au, Kelvin . .P.046 Adams, John T.. P.137 Auer, Iwona . .P.046 Adile, Ashley . .P.044 Auer, Roland . P.113 Afkham, Amir . P.025 Avery, Emma C.. P.014 Agbi, Charles . .P.054, P.055, P.056, P.057 Azarnoush, Hamed. P.079 Agnihotri, Sameer . .P.048 Babinski, Melanie . P.031 Ahmed, Syed Uzair . P.091, P.104, P.126 Badhiwala, Jetan H. P.049 Ahuja, Christopher S.. GP.04, P.086 Bai, Yunfei . P.016 Ajani, Olufemi . P.044 Bajunaid, Khalid . P.079 Al Hashmi, Amal M. .P.090 Baker, Jacquie . .P.066, P.111 Al Mawali, Said . P.090 Balgi, Aruna . P.042 Al Qarni, Mustafa . P.026 Banaz, Fatmahalzahra . .P.055, P.056 Al-Jassim, Abrar . P.031 Bartlett, Peter . P.037 Alan, Hirsch R. ., , , , , , , P.005, P.115 Bass, Ann D. P.027 Alanezi, Ahmad . .P.020 Bastin, Mark E. GP.01 Alawadhi, Abdulla . GP.03, P.101 Becker, Werner. P.024 Aldape, Kenneth . P.051 Bello-Espinosa, Luis . .B.01, P.058, P.060 Alhusaini, Saud . GP.01, P.097 Berkovich, Regina . .P.027 Ali-Ciurana, Yara . P.106 Bertini, Enrico . B.05 Alizadehfar, Reza. GP.03 Bhan, Ishir . .B.05, B.06 AlKhateeb, Mashael. P.061 Bhavsar, Jinil . GP.04 Alkherayf, Fahad . P.053, P.054, P.055, P.056, P.057 Bhullar, Gina . .P.078 Almenawer, Saleh . .P.033, P.098 Bigder, Mark . .P.023, P.126 Almojuela, Alysa . .P.022, P.059 Bitnun, Ari . -
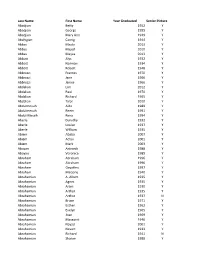
Last Name First Name Year Graduated Senior Picture Abadjian
Last Name First Name Year Graduated Senior Picture Abadjian Betty 1952 Y Abadjian George 1955 Y Abadjian Mary Ann 1949 Y Abahigian Carnig 1944 Y Abbas Majda 2013 Y Abbas Majed 2010 Y Abbas Maysa 2013 Y Abbott Alys 1932 Y Abbott Norman 1934 Y Abbott Robert 1948 Y Abbruzzi Frances 1970 Y Abbruzzi Jane 1966 Y Abbruzzi Janice 1966 Y Abdalian Lori 2012 Y Abdalian Paul 1970 Y Abdalian Richard 1965 Y Abdalian Talar 2010 Y Abdulmessih Aida 1989 Y Abdulmessih Reem 1991 Y Abdul-Messih Rena 1994 Y Aberle Dorothy 1932 Y Aberle Louise 1937 Y Aberle William 1935 Y Abiem Ababa 2007 Y Abiem Achai 2001 Y Abiem Mark 2003 Y Aboyan Antranik 1988 Y Aboyan Veronica 1985 Y Abraham Abraham 1996 Y Abraham Abraham 1996 Y Abraham Gayathry 1997 Y Abraham Marjorie 1940 Y Abrahamian A. Albert 1935 Y Abrahamian Agnes 1935 Y Abrahamian Aram 1930 Y Abrahamian Arthur 1935 Y Abrahamian Arthur 1937 N Abrahamian Bruce 1971 Y Abrahamian Esther 1962 Y Abrahamian Evelyn 1965 Y Abrahamian Joan 1969 Y Abrahamian Margaret 1946 Y Abrahamian Nayad 2001 Y Abrahamian Nevart 1933 Y Abrahamian Richard 1941 N Abrahamian Sharon 1988 Y Last Name First Name Year Graduated Senior Picture Abrahmian David 1971 Y Abramian Jacklin 1979 Y Abramson Barbara L. 1961 Y Abramson Stephen 1968 Y Abreu Antonio 1979 Y Abreu Cheryl 1983 N Abreu Joel 1987 Y Abreu Joseph 1958 Y Abreu Mary 1981 Y Abreu Mary 1982 Y Abreu Ronald 1958 Y Abrillian Garo 1978 Y Aby Laetitia 1998 Y Acaster Claire 1987 Y Acaster Martin 1985 Y Aceto Anthony 1975 Y Aceto Dennis 1972 Y Aceto Diane 1975 Y Aceto Mary 1971 Y Aceto Nancy -

Boston Attorneys
2021 MASSACHUSETTS BAR DIRECTORY BOSTON Explanatory Note: The date immediately following individual names denotes the year of admission to the Massachusetts Bar. For further information about these listings please refer to the alphabetical green pages of this volume. Every effort has been made to obtain accuracy in the listings but the publishers do not guarantee its accuracy. Difficulty is sometimes experienced in securing information. In instances where communications requesting information have elicited no response, the old information has been retained without comment. BOSTON (617) 790-8208..Adams Stephen T ‘96 (617) 727-2200..Aladro Courtney ‘07 (617) 423-0648..Allison Joanna (617) 357-4223..Ancona Christopher Suffolk County (617) 728-0888..Addeo Stephanie ‘19 (617) 526-6000..Alali Arwa ‘18 George ‘95 ‘11 (617) 621-6500..Allison Melissa C ‘04 (617) 742-6720..Andelman David R ‘64 (617) 832-1108..Aarestad Stacie S ‘97 (617) 345-9000..Addesa Christopher M (617) 263-1203..Alanis Elayne ‘05 Nebesky ‘04 (617) 790-3000..Allon Eric R ‘83 Anderman Erin M ‘08 (617) 266-9600..Abae Niloufar ‘19 (617) 777-5936..Addivinola Frank J Jr. (617) 523-6200..Alarcon Erin J McCoy (617) 727-3300..Allonby Judith ‘89 (617) 936-2591..Anderman Jonathan E (617) 330-8272..Abalos Todd J ‘97 ‘09 ‘02 (617) 973-3537..Allouise Patricia A ‘88 ‘08 (617) 704-6663..Abate Chas J Jr. ‘88 (617) 330-1625..Adelson Loria & (877) 552-2529..Alavi + Braza PC (857) 343-0160..Almeida Tyler J ‘18 (617) 951-7000..Andersen Arthur A III (617) 578-3514..Abate Michele Weisman PC (877) -

2008-Dattolo-Luccio-Webist.Pdf
WEBIST 2008 Proceedings of the Fourth International Conference on Web Information Systems and Technologies Volume 1 Funchal, Madeira, Portugal May 4 7, 2008 Co-organized by INSTICC Institute for Systems and Technologies of Information, Control and Communication and UMA Universidade da Madeira Co-sponsored WfMC Workflow Management Coalition I Copyright © 2008 INSTICC Institute for Systems and Technologies of Information, Control and Communication All rights reserved Edited by José Cordeiro, Joaquim Filipe and Slimane Hammoudi Printed in Portugal ISBN: 978-989-8111-26-5 Depósito Legal: 271876/08 http://www.webist.org [email protected] II BRIEF CONTENTS INVITED SPEAKERS........................................................................................................................IV ORGANIZING AND STEERING COMMITTEES .................................................................................... V PROGRAM COMMITTEE .................................................................................................................VI AUXILIARY REVIEWERS ................................................................................................................IX SELECTED PAPERS BOOK ............................................................................................................... X FOREWORD....................................................................................................................................XI CONTENTS................................................................................................................................. -

THE FLAME Sisters of Saint Dominic a Message from the Prioress of Caldwell, NJ
100014_newsltr_newsltr 2/1/13 2:25 PM Page 1 THE Volume 6, No. 1 Winter 2013 FLAME “it seemed to set the earth on fire” CALDWELL DOMINICANS Thank You Friendship, a Dominican Tradition Scripture speaks of holy people as ries. Diana relished their visits so she “friends of God” and surely, we all could hear firsthand the details of the aspire to that title. To be a friend of Mission of which she was a hidden God challenges us in myriad ways. but powerful part! We cannot meet this challenge alone We hear many stories of Albert the –we go to God in communion with Great and his brilliant student, other friends of God! Dominicans Thomas Aquinas. The fact of their cultivate friendships for all the friendship often gets ignored. The human reasons that others do, and two Dominicans were so different: also as a means to grow in faithful- Albert, gregarious, with wide-rang- ness as preachers of the Word. ing interests and experiences; St. Dominic cultivated a deep Thomas, quiet, reflective, almost fruitful friendship with Diego de solitary. Yet Albert called Thomas Avezedo. When Dominic was sub- into community and Thomas through prior of the canons in the Osma his writing put Albert‘s encyclopedic cathedral and Diego was the local knowledge at the service of the bishop, the two trekked together Mission for generations to come. across France on a diplomatic mis- And after Thomas’ death, Albert was sion. As they encountered the people a vehement defender of his friend’s enthralled by the Albigensian heresy, writing. -

2016 Donor's Campaign to End Premature Birth
Campaign to end premature birth The March of Dimes acknowledges a special group of donors who, in 2016, pledged to support the Campaign to End Premature Birth. The campaign is our high-priority, major gifts initiative powered by generous supporters who are part of the movement to unite health care providers, researchers, hospitals, insurers, public health officials, public policy experts and others to realize a future with far fewer preterm births and far more babies born healthy and full term. The March of Dimes is incredibly grateful and recognizes these new pledges and gifts initiated in 2016: Abbott Laboratories, Inc. Dr. Thomas Iannucci Patricia Alridge Ingalls Shipbuilding Kara Altenbaumer-Price Jim Click, Inc. David Batelaan Dr. Richard B. Johnston, Jr. Suzanne Bates Margaret Leigh Kessler Bates Enterprises, Inc. Sashi Kilaru Benevity Ambassador Ron Kirk Vice Admiral Regina M. Benjamin Tom and Susan Koltun Annie Berlin Bob and Laurie Kruchten Dr. David Blann Dr. David L. Lakey Harris Brooks Mark and Jane Landon Jeff Bundy Alyson Layman Steve Burman Gladeline M. Lergier Timothy Butz Brandy and Mark Letson Karen M. Campbell Dr. and Mrs. Charles J. Lockwood David and Kathy Cassady Tom and Dianne Mallini Edward Chien Bernadette Mangan F. Sessions Cole Ron and Cindy Marks Andrew Conrad Dr. James N. Martin Jim Corbett Masimo Corporation Helen Cuccias Dr. Richard and Jo Anne McClead Brad and MacKenzie Curt Faye W. McClure Andy and Kelly Dahle Brian and Mary McGeady DD Traders, Inc. Darryl Miao Gary Dixon The David B. Miller Family Foundation Gary Frank Brent and Eloise Minor Drs. Steven and Patricia Gabbe Jeff and Kelley Minor Alfredo Gangotena Jeff Moore General Motors Financial Company, Inc.