Alkane Alkene Alcohol Ketone Aldehyde Carboxylic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
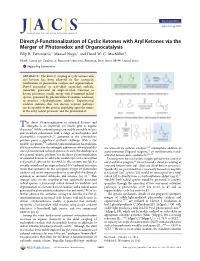
Direct Β‑Functionalization of Cyclic Ketones with Aryl Ketones Via the Merger of Photoredox and Organocatalysis Filip R
Communication pubs.acs.org/JACS Direct β‑Functionalization of Cyclic Ketones with Aryl Ketones via the Merger of Photoredox and Organocatalysis Filip R. Petronijevic,́† Manuel Nappi,† and David W. C. MacMillan* Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl−alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst. he direct β-functionalization of saturated ketones and T aldehydes is an important yet elusive goal in organic chemistry.1 While carbonyl groups are readily amenable to ipso- and α-carbon substitution with a range of nucleophiles and electrophiles respectively,2,3 activation at the β-methylene position poses a significant synthetic challenge. With a few notable exceptions,1,4 carbonyl β-functionalization has tradition- ally been restricted to the conjugate addition of soft nucleophiles are accessed via carbene catalysis,9,10 nucleophilic addition of into α,β-unsaturated carbonyl systems. As such, the development acetal-protected Grignard reagents,11 or stoichiometric metal- 5 − of a general catalytic platform for the direct β-functionalization activated homoenolate equivalents.12 16 of saturated ketones or aldehydes would represent a conceptual Drawing from the mechanistic insights gained in the course of and practical advance for the field. -

The Reaction of Aminonitriles with Aminothiols: a Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean
life Article The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean Ibrahim Shalayel , Seydou Coulibaly, Kieu Dung Ly, Anne Milet and Yannick Vallée * Univ. Grenoble Alpes, CNRS, Département de Chimie Moléculaire, Campus, F-38058 Grenoble, France; [email protected] (I.S.); [email protected] (S.C.); [email protected] (K.D.L.); [email protected] (A.M.) * Correspondence: [email protected] Received: 28 September 2018; Accepted: 18 October 2018; Published: 19 October 2018 Abstract: The Strecker reaction of aldehydes with ammonia and hydrogen cyanide first leads to α-aminonitriles, which are then hydrolyzed to α-amino acids. However, before reacting with water, these aminonitriles can be trapped by aminothiols, such as cysteine or homocysteine, to give 5- or 6-membered ring heterocycles, which in turn are hydrolyzed to dipeptides. We propose that this two-step process enabled the formation of thiol-containing dipeptides in the primitive ocean. These small peptides are able to promote the formation of other peptide bonds and of heterocyclic molecules. Theoretical calculations support our experimental results. They predict that α-aminonitriles should be more reactive than other nitriles, and that imidazoles should be formed from transiently formed amidinonitriles. Overall, this set of reactions delineates a possible early stage of the development of organic chemistry, hence of life, on Earth dominated by nitriles and thiol-rich peptides (TRP). Keywords: origin of life; prebiotic chemistry; thiol-rich peptides; cysteine; aminonitriles; imidazoles 1. Introduction In ribosomes, peptide bonds are formed by the reaction of the amine group of an amino acid with an ester function. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Aldehydes and Ketones Are Simple Organic Compounds Containing a Carbonyl Group
Aldehydes and Ketones are simple organic compounds containing a carbonyl group. Carbonyl group contains carbon- oxygen double bond. These organic compounds are simple because the carbon atom presents in the carbonyl group lack reactive groups such as OH or Cl. By Dr. Sayed Hasan Mehdi Assistant Professor Department of Chemistry Shia P.G. College, Lucknow Dr. S.Hasan Mehdi 6/13/2020 This is to bring to kind notice that the matter of this e- content is for the B.Sc. IV semester students. It has been taken from the following sources. The students are advised to follow these books as well. •A TEXTBOOK OF ORGANIC CHEMISTRY by Arun Bahl & B.S. Bahl, S. Chand & Company Ltd. Publication. •Graduate Organic Chemistry by M. K. Jain and S.C. Sharma, Vishal Publishing Co. •Pradeep’s Organic Chemistry Vol II by R. N. Dhawan, Pradeep Publication, Jalandhar. Dr. S.Hasan Mehdi 6/13/2020 An aldehyde is one of the classes of carbonyl group- containing alkyl group on one end and hydrogen on the other end. The R and Ar denote alkyl or aryl member respectively. In the condensed form, the aldehyde is written as –CHO. Dr. S.Hasan Mehdi 6/13/2020 Dr. S.Hasan Mehdi 6/13/2020 1. From Alcohols: a. By oxidation of Alcohols: Aldehydes and ketones can be prepared by the controlled oxidation of primary and secondary alcohols using an acidified solution of potassium dichromate or permanganate. Primary alcohol produces aldehydesRef. Last slide. O K Cr O RCH2OH + [O] 2 2 7 + R C H H 10 Alcohol Aldehyde O CH3CH2OH+ [O] K2Cr2O7 + CH3 C H H Ethyl Alcohol Acetaldehyde The aldehydes formed in the above reaction are very easily oxidised to carboxylc acids if allowed to remain in the reaction mixture. -

3-Monochloropropane-1,2-Diol Esters and Glycidyl Esters
www.nature.com/scientificreports OPEN Monitoring of heat‑induced carcinogenic compounds (3‑monochloropropane‑1,2‑diol esters and glycidyl esters) in fries Yu Hua Wong1, Kok Ming Goh1,2, Kar Lin Nyam2, Ling Zhi Cheong3, Yong Wang4, Imededdine Arbi Nehdi5,6, Lamjed Mansour7 & Chin Ping Tan1* 3‑Monochloropropane‑1,2‑diol (3‑MCPD) esters and glycidyl esters (GE) are heat‑induced contaminants which form during oil refning process, particularly at the high temperature deodorization stage. It is worth to investigate the content of 3‑MCPD and GE in fries which also involved high temperature. The content of 3‑MCPD esters and GE were monitored in fries. The factors that been chosen were temperature and duration of frying, and diferent concentration of salt (NaCl). The results in our study showed that the efect was in the order of concentration of sodium chloride < frying duration < frying temperature. The content of 3‑MCPD esters was signifcantly increased whereas GE was signifcantly decreased, when prolong the frying duration. A high temperature results in a high 3‑MCPD ester level but a low GE level in fries. The present of salt had contributed signifcant infuence to the generation of 3‑MCPD. The soaking of potato chips in salt showed no signifcant efect on the level of GE during the frying. The oil oxidation tests showed that all the fries were below the safety limit. Hence, the frying cycle, temperature and the added salt to carbohydrate‑based food during frying should be monitored. Deep-fat frying is commonly being used to process food. During the process, heat transfer between the fried food and oil is occurs. -

Aldehydes and Ketone
ALDEHYDES AND KETONE www.gneet.com ALDEHYDES AND KETONES In aldehydes, the carbonyl group is linked to either two hydrogen atom or one hydrogen atom and one carbon containing group such as alkyl, aryl or aralkyl group Examples * In ketones, the carbonyl group is linked to two carbon containing groups which may be same or different alkyl, aryl group. If two R and R’ groups are same, the ketone is called simple or symmetrical ketone and if R and R’ are different, then ketone is known as mixed or an unsymmetrical ketone. STRUCTURE Carbonyl carbon of both aldehyde and ketones is sp2 – hybridised, One of the three sp2 hybridised orbital get involved in σ- bond formation with half –filled p-orbital of oxygen atom whereas rest of the two are consumed in σ-bond formation with hydrogen and carbon depending on the structure of aldehyde or ketone. Unhybridised p-orbital of carbonyl carbon form π-bond with another half-filled p-orbital of oxygen atom by sideways overlapping. ISOMERISM IN ALDEHYDES AND KETONES (a) Chain isomerism: Aldehydes ( with 4 or more carbon atoms) and ketone ( with 5 or more carbon atoms) show chain isomerism. Example i) C4H8O CH3-CH2-CH2-CHO ( butanal) 1 ALDEHYDES AND KETONE www.gneet.com ii) C5H10O (b) Position isomerism: aliphatic aldehydes do not show position isomerism, because –CHO group is always present at the end of carbon chain. Aromatic aldehyde show position isomerism. Example (c) Metamerism: Higher ketones show metamerism due to presence of different alkyl groups attached to the same functional group C5H10O (d) Functional isomerism : Aldehydes and ketones show functional isomerism in them. -

Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles
Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles Oliver J. Turner,*[a,b] John A. Murphy,*[b] David. J. Hirst[a] and Eric P. A. Talbot*[a]† Abstract: Hydrogen atom transfer-mediated intramolecular C-C coupling reactions between alkenes and nitriles, using PhSiH3 and catalytic Fe(acac)3, are described. This introduces a new strategic bond disconnection for ring-closing reactions, forming ketones via imine intermediates. Of note is the scope of the reaction, including formation of sterically hindered ketones, spirocycles and fused cyclic systems. In the early 1960s, Kwiatek and Seyler first reported the use of metal hydrides as catalysts in the hydrogenation of α,β- unsaturated compounds.[1,2] The discovery by Halpern,[3] later elegantly developed by Norton,[4] that metal-hydride hydrogen atom transfer (HAT) proceeded by a free-radical mechanism opened the door to a wide range of alkene hydrofunctionalisation reactions. But it was the pioneering work by Mukaiyama[5] on the catalytic hydration of alkenes, using Co(acac)2 and oxygen, that sparked wider interest in the field of alkene hydrofunctionalisation. As a result, there now exists an extensive ‘toolkit’ for the addition of hydrogen and a functional group to an alkene with Markovnikov selectivity and high chemo-selectivity using cobalt, manganese and iron complexes.[6,7] Efforts have also been made to extend HAT methodologies to C-C bond formation, both in an intra- and intermolecular fashion: Baran’s group developed a general C-C coupling reaction, utilising electron-deficient alkenes as capable radical acceptors (Scheme 1ai).[8–10] Hydropyridylation of alkenes by intramolecular Minisci reaction was recently demonstrated by Starr,[11] which allows for the formation of structures such as Scheme 1. -

Nitrile Synthesis with Aldoxime Dehydratases: a Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries
molecules Review Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries Pablo Domínguez de María Sustainable Momentum, SL, Av. Ansite 3, 4–6, 35011 Las Palmas de Gran Canaria, Canary Islands, Spain; [email protected]; Tel.: +34-6-0956-5237 Abstract: Nitriles comprise a broad group of chemicals that are currently being industrially produced and used in fine chemicals and pharmaceuticals, as well as in bulk applications, polymer chemistry, solvents, etc. Aldoxime dehydratases catalyze the cyanide-free synthesis of nitriles starting from aldoximes under mild conditions, holding potential to become sustainable alternatives for industrial processes. Different aldoxime dehydratases accept a broad range of aldoximes with impressive high substrate loadings of up to >1 Kg L−1 and can efficiently catalyze the reaction in aqueous media as well as in non-aqueous systems, such as organic solvents and solvent-free (neat substrates). This paper provides an overview of the recent developments in this field with emphasis on strategies that may be of relevance for industry and sustainability. When possible, potential links to biorefineries and to the use of biogenic raw materials are discussed. Keywords: biocatalysis; green chemistry; nitriles; aldoxime dehydratases; sustainability Citation: Domínguez de María, P. Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk 1. Aldoxime Dehydratases as Biocatalysts for the Cyanide-Free Synthesis of Nitriles Chemicals, and Biorefineries. Nitriles comprise an important group of chemicals that are widely spread in industry Molecules 2021, 26, 4466. in a broad range of sectors, being used as products, solvents, polymers, commodities, or https://doi.org/10.3390/ as starting materials for the production of other chemicals such as amines, amides, etc. -

Chapter 14 – Aldehydes and Ketones
Chapter 14 – Aldehydes and Ketones 14.1 Structures and Physical Properties of Aldehydes and Ketones Ketones and aldehydes are related in that they each possess a C=O (carbonyl) group. They differ in that the carbonyl carbon in ketones is bound to two carbon atoms (RCOR’), while that in aldehydes is bound to at least one hydrogen (H2CO and RCHO). Thus aldehydes always place the carbonyl group on a terminal (end) carbon, while the carbonyl group in ketones is always internal. Some common examples include (common name in parentheses): O O H HH methanal (formaldehyde) trans-3-phenyl-2-propenal (cinnamaldehyde) preservative oil of cinnamon O O propanone (acetone) 3-methylcyclopentadecanone (muscone) nail polish remover a component of one type of musk oil Simple aldehydes (e.g. formaldehyde) typically have an unpleasant, irritating odor. Aldehydes adjacent to a string of double bonds (e.g. 3-phenyl-2-propenal) frequently have pleasant odors. Other examples include the primary flavoring agents in oil of bitter almond (Ph- CHO) and vanilla (C6H3(OH)(OCH3)(CHO)). As your book says, simple ketones have distinctive odors (similar to acetone) that are typically not unpleasant in low doses. Like aldehydes, placing a collection of double bonds adjacent to a ketone carbonyl generally makes the substance more fragrant. The primary flavoring agent in oil of caraway is just a such a ketone. 2 O oil of carraway Because the C=O group is polar, small aldehydes and ketones enjoy significant water solubility. They are also quite soluble in typical organic solvents. 14.2 Naming Aldehydes and Ketones Aldehydes The IUPAC names for aldehydes are obtained by using rules similar to those we’ve seen for other functional groups (e.g. -

Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani
Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani To cite this version: Rim Mouselmani. Reduction of Organic Functional Groups Using Hypophosphites. Other. Univer- sité de Lyon; École Doctorale des Sciences et de Technologie (Beyrouth), 2018. English. NNT : 2018LYSE1241. tel-02147583v2 HAL Id: tel-02147583 https://tel.archives-ouvertes.fr/tel-02147583v2 Submitted on 5 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE de DOCTORAT DE L’UNIVERSITE DE LYON EN COTUTELLE AVEC L'UNIVERSITÉ LIBANAISE opérée au sein de l’Université Claude Bernard Lyon 1 École Doctorale de Chimie-École Doctorale des Sciences et Technologies Discipline : Chimie Soutenue publiquement le 07/11/2018, par Rim MOUSELMANI Reduction of Organic Functional Groups Using Hypophosphites Devant le jury composé de Mme. Micheline DRAYE Université Savoie Mont Blanc Rapporteure M. Mohammad ELDAKDOUKI Université Arabe de Beyrouth Rapporteur Mme. Emmanuelle SCHULZ Université Paris 11 examinatrice M. Abderrahmane AMGOUNE Université Lyon 1 Président M. Mahmoud FARAJ Université Internationale Libanaise examinateur Mme. Estelle MÉTAY Université Lyon 1 Directrice de thèse M. Ali HACHEM Université Libanaise Directeur de thèse M. Marc LEMAIRE Université Lyon 1 Membre invité M. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Than Was the Monoleucyl Ester of Cis-Cyclopentane-1,2-Diol
SOME OBSERVATIONS ON THE MECHANISM OF THE ACYLATION PROCESS IN PROTEIN SYNTHESIS* BY BEVERLY E. GRIFFIN AND C. B. REESE UNIVERSITY CHEMICAL LABORATORY, CAMBRIDGE, ENGLAND Communicated by Lord Todd, January 2, 1964 During recent years much attention has been given to the mechanism of synthesis of proteins in biological systems.' Although the process is by no means fully understood, some of the steps involved appear to be well established.2-4 Before amino acids can be incorporated into polypeptide chains they must first be "activated." The "activation" process involves ribonucleic acids of comparatively low molecular weight, known as "transfer" or "soluble" ribonucleic acids (sRNA), adenosine-5' triphosphate (ATP) as an energy source, and a specific enzyme for each amino acid. In the first step of the "activation" process, the amino acid reacts with ATP to form a mixed anhydride with adenosine-5' phosphate (AMP) releasing inorganic pyrophosphate; this mixed anhydride then acylates a specific sRNA on its terminal nucleoside (adenosine) residue to yield a 2' (or 3')-aminoacyl ester-the "activated" amino-acid. These processes are reversible. ATP + amino acid aminoacyl-AMP + pyrophosphate (1) sRNA + aminoacyl-AMP aminoacyl-sRNA + AMP (2) Beyond this point our knowledge of the process of polypeptide synthesis is less certain. The "activated" amino acids are believed to be transferred to the ribo- somes-the site of assembly of polypeptide chains-and then the amino acids become linked together in a genetically controlled order to synthesize a specific protein.5 Although there is as yet no definite evidence regarding this final step, it is frequently assumed that it involves a simple acylation as indicated in step (3).