4-Phenyl-1,2,4-Triazoline-3,5-Dione in the Ene Reactions with Cyclohexene, 1-Hexene and 2, 3-Dimethyl-2-Butene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intramolecular Ene Reactions of Functionalised Nitroso Compounds
INTRAMOLECULAR ENE REACTIONS OF FUNCTIONALISED NITROSO COMPOUNDS A Thesis Presented by Sandra Luengo Arratta In Partial Fulfilment of the Requirements for the Award of the Degree of DOCTOR OF PHILOSOPHY OF UNIVERSITY COLLEGE LONDON Christopher Ingold Laboratories Department of Chemistry University College London 20 Gordon Street London WC1H 0AJ July 2010 DECLARATION I Sandra Luengo Arratta, confirm that the work presented in this thesis is my own. Where work has been derived from other sources, I confirm that this has been indicated in the thesis. ABSTRACT This thesis concerns the generation of geminally functionalised nitroso compounds and their subsequent use in intramolecular ene reactions of types I and II, in order to generate hydroxylamine derivatives which can evolve to the corresponding nitrones. The product nitrones can then be trapped in the inter- or intramolecular mode by a variety of reactions, including 1,3-dipolar cycloadditions, thereby leading to diversity oriented synthesis. The first section comprises the chemistry of the nitroso group with a brief discussion of the current methods for their generation together with the scope and limitations of these methods for carrying out nitroso ene reactions, with different examples of its potential as a powerful synthetic method to generate target drugs. The second chapter describes the results of the research programme and opens with the development of methods for the generation of functionalised nitroso compounds from different precursors including oximes and nitro compounds, using a range of reactants and conditions. The application of these methods in intramolecular nitroso ene reactions is then discussed. Chapter three presents the conclusions which have been drawn from the work presented in chapter two, and provides suggestions for possible directions of this research in the future. -

Appendix I: Named Reactions Single-Bond Forming Reactions Co
Appendix I: Named Reactions 235 / 335 432 / 533 synthesis / / synthesis Covered in Covered Featured in problem set problem Single-bond forming reactions Grignard reaction various Radical couplings hirstutene Conjugate addition / Michael reaction strychnine Stork enamine additions Aldol-type reactions (incl. Mukaiyama aldol) various (aldol / Claisen / Knoevenagel / Mannich / Henry etc.) Asymmetric aldol reactions: Evans / Carreira etc. saframycin A Organocatalytic asymmetric aldol saframycin A Pseudoephedrine glycinamide alkylation saframycin A Prins reaction Prins-pinacol reaction problem set # 2 Morita-Baylis-Hillman reaction McMurry condensation Gabriel synthesis problem set #3 Double-bond forming reactions Wittig reaction prostaglandin Horner-Wadsworth-Emmons reaction prostaglandin Still-Gennari olefination general discussion Julia olefination and heteroaryl variants within the Corey-Winter olefination prostaglandin Peterson olefination synthesis Barton extrusion reaction Tebbe olefination / other methylene-forming reactions tetrodotoxin hirstutene / Selenoxide elimination tetrodotoxin Burgess dehydration problem set # 3 Electrocyclic reactions and related transformations Diels-Alder reaction problem set # 1 Asymmetric Diels-Alder reaction prostaglandin Ene reaction problem set # 3 1,3-dipolar cycloadditions various [2,3] sigmatropic rearrangement various Cope rearrangement periplanone Claisen rearrangement hirstutene Oxidations – Also See Handout # 1 Swern-type oxidations (Swern / Moffatt / Parikh-Doering etc. N1999A2 Jones oxidation -

Steam Cracking: Chemical Engineering
Steam Cracking: Kinetics and Feed Characterisation João Pedro Vilhena de Freitas Moreira Thesis to obtain the Master of Science Degree in Chemical Engineering Supervisors: Professor Doctor Henrique Aníbal Santos de Matos Doctor Štepánˇ Špatenka Examination Committee Chairperson: Professor Doctor Carlos Manuel Faria de Barros Henriques Supervisor: Professor Doctor Henrique Aníbal Santos de Matos Member of the Committee: Specialist Engineer André Alexandre Bravo Ferreira Vilelas November 2015 ii The roots of education are bitter, but the fruit is sweet. – Aristotle All I am I owe to my mother. – George Washington iii iv Acknowledgments To begin with, my deepest thanks to Professor Carla Pinheiro, Professor Henrique Matos and Pro- fessor Costas Pantelides for allowing me to take this internship at Process Systems Enterprise Ltd., London, a seven-month truly worthy experience for both my professional and personal life which I will certainly never forget. I would also like to thank my PSE and IST supervisors, who help me to go through this final journey as a Chemical Engineering student. To Stˇ epˇ an´ and Sreekumar from PSE, thank you so much for your patience, for helping and encouraging me to always keep a positive attitude, even when harder problems arose. To Prof. Henrique who always showed availability to answer my questions and to meet in person whenever possible. Gostaria tambem´ de agradecer aos meus colegas de casa e de curso Andre,´ Frederico, Joana e Miguel, com quem partilhei casa. Foi uma experienciaˆ inesquec´ıvel que atravessamos´ juntos e cer- tamente que a vossa presenc¸a diaria´ apos´ cada dia de trabalho ajudou imenso a aliviar as saudades de casa. -

Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters
Master in Chemical Engineering Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters Thesis of the Master’s Degree Development Project in Foreign Environment Cláudia Sofia Martins Angeira Erasmus coordinator: Eng. Miguel Madeira Examiner in FEUP: Eng. Fernando Martins Supervisor in ICT: Doc. Ing Petr Zámostný July 2008 Acknowledgements I would like to thank Doc. Ing. Petr Zámostný for the opportunity to hold a master's thesis in this project, the orientation, the support given during the laboratory work and suggestions for improvement through the work. i Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters Abstract This research deals with the study of hydrocarbon thermal cracking with the aim of producing ethylene, one of the most important raw materials in Chemical Industry. The main objective was the study of cracking reactions of hydrocarbons by means of measuring the selectivity of hydrocarbons primary cracking and evaluating the relationship between the structure and the behavior. This project constitutes one part of a bigger project involving the study of more than 30 hydrocarbons with broad structure variability. The work made in this particular project was focused on the study of the double bond position effect in linear unsaturated hydrocarbons. Laboratory experiments were carried out in the Laboratory of Gas and Pyrolysis Chromatography at the Department of Organic Technology, Institute of Chemical Technology, Prague, using for all experiments the same apparatus, Pyrolysis Gas Chromatograph, to increase the reliability and feasibility of results obtained. Linear octenes with different double bond position in hydrocarbon chain were used as model compounds. In order to achieve these goals, the primary cracking reactions were studied by the method of primary selectivities. -

Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes
Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes Stephen Goble Organic Super-Group Meeting Literature Presentation October 6, 2004 1 Outline I. Background • Introduction to Carbometallation • “Inner Sphere” vs. “Outer Sphere” • Review of Seminal Work II. Catalytic Palladium Systems III. Catalytic Platinum Systems IV. Catalytic Gold Systems 2 I. Background: Carbometallation is the formal addition of a metal and a carbon atom across a double bond. • Cis addition would involve olefin insertion in to an σ-alkyl-metal species. This is an “Inner Coordination Sphere” process. cis addition [M] [M] R olefin insertion R • The alkyl-metal species could arise from: 1. Oxidative addition of the metal to an electrophile. 2. Transmetallation of a nucleophile to the metal. 3. Direct nucleophilic attack on the palladium. • Trans addition would involve an “Outer Coordination Sphere” nucleophilic attack on the metal-olefin coordination complex. trans addition [M] [M] R- R 3 First Example: Carbopalladation of an Olefin Tsuji, J. and Takahashi, H. J. Am. Chem. Soc. 1965. 87(14), 3275-3276. [Pd] O O RO2C CO R PdCl2 RO OR 2 Na2CO3 [Pd] Cl • Amino and Oxo-palladations (i.e. Wacker Process) were previously known and have since been much more widely studied. • No distinction between cis and trans nucleophilic addition made. 4 Carbopalladation on Styrene: Different Modes of Attack Proposed different modes of attack based on different observed products with MeLi vs. Na-Malonate: Murahashi, S. et. al. J. Org. Chem. 1977. 42 (17), 2870-2874. Nucleophilic Attack on Pd Olefin Insertion B-Hydride Elimination CH3Li [Pd] [Pd] CH3 PdX2 CH3 [Pd]-H Inner Sphere Mechanism Palladium Coordination Nucleophilic Attack B-hydride elimination CO R CO2R 2 NaCH(CO2R) CO R [Pd] CO2R 2 PdX2 [Pd] [Pd]-H • Outer Sphere attack usually occurs at the more substituted carbon - more stabilization of + + charge (In intermolecular processes). -

Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals
Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Ho, Chun-Yu, Kristin Schleicher, Chun-Wa Chan, and Timothy Jamison. “Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals.” Synlett 2009, no. 16 (October 4, 2009): 2565-2582. As Published http://dx.doi.org/10.1055/s-0029-1217747 Publisher Thieme Publishing Group Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/82119 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ NIH Public Access Author Manuscript Synlett. Author manuscript; available in PMC 2011 September 6. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Synlett. 2009 October 1; 2009(16): 2565±2582. doi:10.1055/s-0029-1217747. Catalytic Addition of Simple Alkenes to Carbonyl Compounds Using Group 10 Metals Chun-Yu Hoa, Kristin D. Schleicherb, and Timothy F. Jamisonb Chun-Yu Ho: [email protected]; Timothy F. Jamison: [email protected] a Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR (P.R. China), Fax: (852) 2603-5057 b Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 Abstract Recent advances using nickel complexes in the activation of unactivated monosubstituted olefins for catalytic intermolecular carbon–carbon bond-forming reactions with carbonyl compounds, such as simple aldehydes, isocyanates, and conjugated aldehydes and ketones, are discussed. -

Named Organic Reactions
Named Organic Reactions Thomas Laue and Andreas Piagens Technical University, Braunschweig, Germany Translated into English by Dr. Claus Vogel Universität Magdeburg, Germany JOHN WILEY & SONS Chichester • New York • Weinheim • Brisbane • Singapore • Toronto Contents Introduction ix Acyloin Ester Condensation 1 Aldol Reaction 4 Alkene Metathesis 10 Arbuzov Reaction 12 Arndt-Eistert Synthesis 13 Baeyer-Villiger Oxidation 16 Bamford-Stevens Reaction 19 Beckmann Rearrangement 22 Benzidine Rearrangement 24 Benzilic Acid Rearrangement 26 Benzoin Condensation 27 Bergman Cyclization 29 Birch Reduction 33 Blanc Reaction 36 Bucherer Reaction 37 Cannizzaro Reaction 40 Chugaev Reaction 42 Claisen Ester Condensation 45 Claisen Rearrangement 48 Clemmensen Reduction 52 Cope Elimination Reaction 54 Cope Rearrangement 56 Corey-Winter Fragmentation 59 Curtius Reaction 61 1,3-Dipolar Cycloaddition 64 [2 + 2] Cycloaddition 67 Darzens Glycidic Ester Condensation 71 Delepine Reaction 73 Diazo Coupling 74 Diazotization 77 Diels-Alder Reaction 78 vi Contents Di-7r-Methane Rearrangement 86 Dötz Reaction 88 Elbs Reaction 92 Ene Reaction 93 Ester Pyrolysis 97 Favorskii Rearrangement 100 Finkelstein Reaction 102 Fischer Indole Synthesis 103 Friedel-Crafts Acylation 106 Friedel-Crafts Alkylation 110 Friedländer Quinoline Synthesis 114 Fries Rearrangement 116 Gabriel Synthesis 120 Gattermann Synthesis 123 Glaser Coupling Reaction 125 Glycol Cleavage 127 Gomberg-B achmann Reaction 129 Grignard Reaction 132 Haloform Reaction 139 Hantzsch Pyridine Synthesis 141 Heck Reaction -

C9sc03756j1.Pdf
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information for Orthogonal Functionalization of Alternating Polyesters: Selective Patterning of (AB)n Sequences Ni Yi,a† Thomas T. D. Chen,a† Junjuda Unruangsri,b Yunqing Zhu,b and Charlotte K. Williamsa* a Chemistry researCh laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK b Department of Chemistry, Imperial College London, London, SW7 2AZ, UK †Joint first authors [email protected] TaBle of Contents SeCtion, Scheme, Table or Figure Pages Experimental SeCtion 3-6 Figure S1. StruCtures of the alternating polyesters P1-P9, all featuring internal and 4 terminal alkene funCtionalities. TaBle S1. Composition and Properties of P1-P9. 5 Scheme S1. One-pot hydroboration-oxidation reaCtion to Convert the polyester 5 terminal alkenes to alternating hydroxyl groups. Scheme S2. Photo-initiated thiol-ene reaCtions to transform the hydroxyl- 6 funCtionalized alternating polyesters into orthogonally funCtionalized (AB)n Polyesters TaBle S2. Preparation Data for Polymers P1(B) - P1(f) and data to aCCompany in Table 6 1. Figure S2. NMR speCtra for P1. 7 Figure S3. 2D NMR speCtra for P1. 8 1 Figure S4. H NMR speCtrum of polymer P1 (CDCl3, 500.0 MHz, 298 K). 9 13 1 Figure S5. C{ H} NMR speCtrum of P1 (d6-DMSO, 500.0 MHz, 298 K). 9 Figure S6. MALDI-ToF speCtrum of P1 synthesized with CHD as the CTA, (Table S1, #1). 10 TaBle S3. SEC CharaCterization data (in THF and DMF) for P1, P2 and P3. -

Advanced Organic Chemistry PART A: Structure and Mechanisms PART B: Reactions and Synthesis Advanced Organic FIFTH Chemistry EDITION
Advanced Organic FIFTH Chemistry EDITION Part B: Reactions and Synthesis Advanced Organic Chemistry PART A: Structure and Mechanisms PART B: Reactions and Synthesis Advanced Organic FIFTH Chemistry EDITION Part B: Reactions and Synthesis FRANCIS A. CAREY and RICHARD J. SUNDBERG University of Virginia Charlottesville, Virginia 123 Francis A. Carey Richard J. Sundberg Department of Chemistry Department of Chemistry University of Virginia University of Virginia Charlottesville, VA 22904 Charlottesville, VA 22904 Library of Congress Control Number: 2006939782 ISBN-13: 978-0-387-68350-8 (hard cover) e-ISBN-13: 978-0-387-44899-2 ISBN-13: 978-0-387-68354-6 (soft cover) Printed on acid-free paper. ©2007 Springer Science+Business Media, LLC All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now know or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. 98765432(corrected 2nd printing, 2008) springer.com Preface The methods of organic synthesis have continued to advance rapidly and we have made an effort to reflect those advances in this Fifth Edition. -

Name Reactions
Name Reactions A Collection of Detailed Reaction Mechanisms Bearbeitet von Jie Jack Li 1. Auflage 2002. Buch. xviii, 417 S. Hardcover ISBN 978 3 540 43024 7 Format (B x L): 15,5 x 23,5 cm Gewicht: 780 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Chemie Allgemein Zu Leseprobe schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Table of Contents Preface ................................................................................................................XIII Abbreviations.......................................................................................................XV 1. Abnormal Claisen rearrangement..............................................................1 2. Alder ene reaction......................................................................................2 3. Allan–Robinson reaction ...........................................................................3 4. Alper carbonylation...................................................................................5 5. Amadori glucosamine rearrangement........................................................7 6. Angeli–Rimini hydroxamic acid synthesis................................................8 -

The Merck Index
Browse Organic Name Reactions ● Preface ● 4CC ● Acetoacetic Ester Condensation ● Acetoacetic Ester Synthesis ● Acyloin Condensation ● Addition ● Akabori Amino Acid Reactions ● Alder (see Diels-Alder Reaction) ● Alder-Ene Reaction ● Aldol Reaction (Condensation) ● Algar-Flynn-Oyamada Reaction ● Allan-Robinson Reaction ● Allylic Rearrangements ● Aluminum Alkoxide Reduction ● Aluminum Alkoxide Reduction (see Meerwein-Ponndorf-Verley Reduction) ● Amadori Rearrangement ● Amidine and Ortho Ester Synthesis ● Aniline Rearrangement ● Arbuzov (see Michaelis-Arbuzov Reaction) ● Arens-van Dorp Synthesis ● Arndt-Eistert Synthesis ● Auwers Synthesis ● Babayan (see Favorskii-Babayan Synthesis) ● Bachmann (see Gomberg-Bachmann Reaction) ● Bäcklund (see Ramberg-Bäcklund Reaction) ● Baeyer-Drewson Indigo Synthesis ● Baeyer-Villiger Reaction ● Baker-Venkataraman Rearrangement ● Bakshi (see Corey-Bakshi-Shibata Reduction) ● Balz-Schiemann Reaction ● Bamberger Rearrangement ● Bamford-Stevens Reaction ● Barbier(-type) Reaction ● Barbier-Wieland Degradation ● Bart Reaction ● Barton Decarboxylation ● Barton Deoxygenation ● Barton Olefin Synthesis ● Barton Reaction http://themerckindex.cambridgesoft.com/TheMerckIndex/NameReactions/TOC.asp (1 of 17)19/4/2005 20:00:21 Browse Organic Name Reactions ● Barton-Kellogg Reaction ● Barton-McCombie Reaction ● Barton-Zard Reaction ● Baudisch Reaction ● Bauer (see Haller-Bauer Reaction) ● Baumann (see Schotten-Baumann Reaction) ● Baylis-Hillman Reaction ● Béchamp Reduction ● Beckmann Fragmentation ● Beckmann Rearrangement -
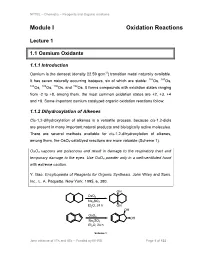
Module I Oxidation Reactions
NPTEL – Chemistry – Reagents and Organic reactions Module I Oxidation Reactions Lecture 1 1.1 Osmium Oxidants 1.1.1 Introduction Osmium is the densest (density 22.59 gcm-3) transition metal naturally available. It has seven naturally occurring isotopes, six of which are stable: 184Os, 187Os, 188Os, 189Os, 190Os, and 192Os. It forms compounds with oxidation states ranging from -2 to +8, among them, the most common oxidation states are +2, +3, +4 and +8. Some important osmium catalyzed organic oxidation reactions follow: 1.1.2 Dihydroxylation of Alkenes Cis-1,2-dihydroxylation of alkenes is a versatile process, because cis-1,2-diols are present in many important natural products and biologically active molecules. There are several methods available for cis-1,2-dihydroxylation of alkenes, among them, the OsO4-catalzyed reactions are more valuable (Scheme 1). OsO4 vapours are poisonous and result in damage to the respiratory tract and temporary damage to the eyes. Use OsO4 powder only in a well-ventilated hood with extreme caution. Y. Gao, Encylcopedia of Reagents for Organic Synthesis, John Wiley and Sons, Inc., L. A. Paquette, New York, 1995, 6, 380. OH OsO4 Na2SO3 Et2O, 24 h OH OH OsO4 OH Na2SO3 Et O, 24 h 2 Scheme 1 Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 122 NPTEL – Chemistry – Reagents and Organic reactions The use of tertiary amine such as triethyl amine or pyridine enhances the rate of reaction (Scheme 2). OH OsO4, Pyridine K2CO3, KOH OH Et O, 30 min 2 Scheme 2 Catalytic amount of OsO4 can be used along with an oxidizing agent, which oxidizes the reduced osmium(VI) into osmium(VIII) to regenerate the catalyst.