Named Reactions Single-Bond Forming Reactions Cov Ered in 2 Y
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intramolecular Ene Reactions of Functionalised Nitroso Compounds
INTRAMOLECULAR ENE REACTIONS OF FUNCTIONALISED NITROSO COMPOUNDS A Thesis Presented by Sandra Luengo Arratta In Partial Fulfilment of the Requirements for the Award of the Degree of DOCTOR OF PHILOSOPHY OF UNIVERSITY COLLEGE LONDON Christopher Ingold Laboratories Department of Chemistry University College London 20 Gordon Street London WC1H 0AJ July 2010 DECLARATION I Sandra Luengo Arratta, confirm that the work presented in this thesis is my own. Where work has been derived from other sources, I confirm that this has been indicated in the thesis. ABSTRACT This thesis concerns the generation of geminally functionalised nitroso compounds and their subsequent use in intramolecular ene reactions of types I and II, in order to generate hydroxylamine derivatives which can evolve to the corresponding nitrones. The product nitrones can then be trapped in the inter- or intramolecular mode by a variety of reactions, including 1,3-dipolar cycloadditions, thereby leading to diversity oriented synthesis. The first section comprises the chemistry of the nitroso group with a brief discussion of the current methods for their generation together with the scope and limitations of these methods for carrying out nitroso ene reactions, with different examples of its potential as a powerful synthetic method to generate target drugs. The second chapter describes the results of the research programme and opens with the development of methods for the generation of functionalised nitroso compounds from different precursors including oximes and nitro compounds, using a range of reactants and conditions. The application of these methods in intramolecular nitroso ene reactions is then discussed. Chapter three presents the conclusions which have been drawn from the work presented in chapter two, and provides suggestions for possible directions of this research in the future. -

Appendix I: Named Reactions Single-Bond Forming Reactions Co
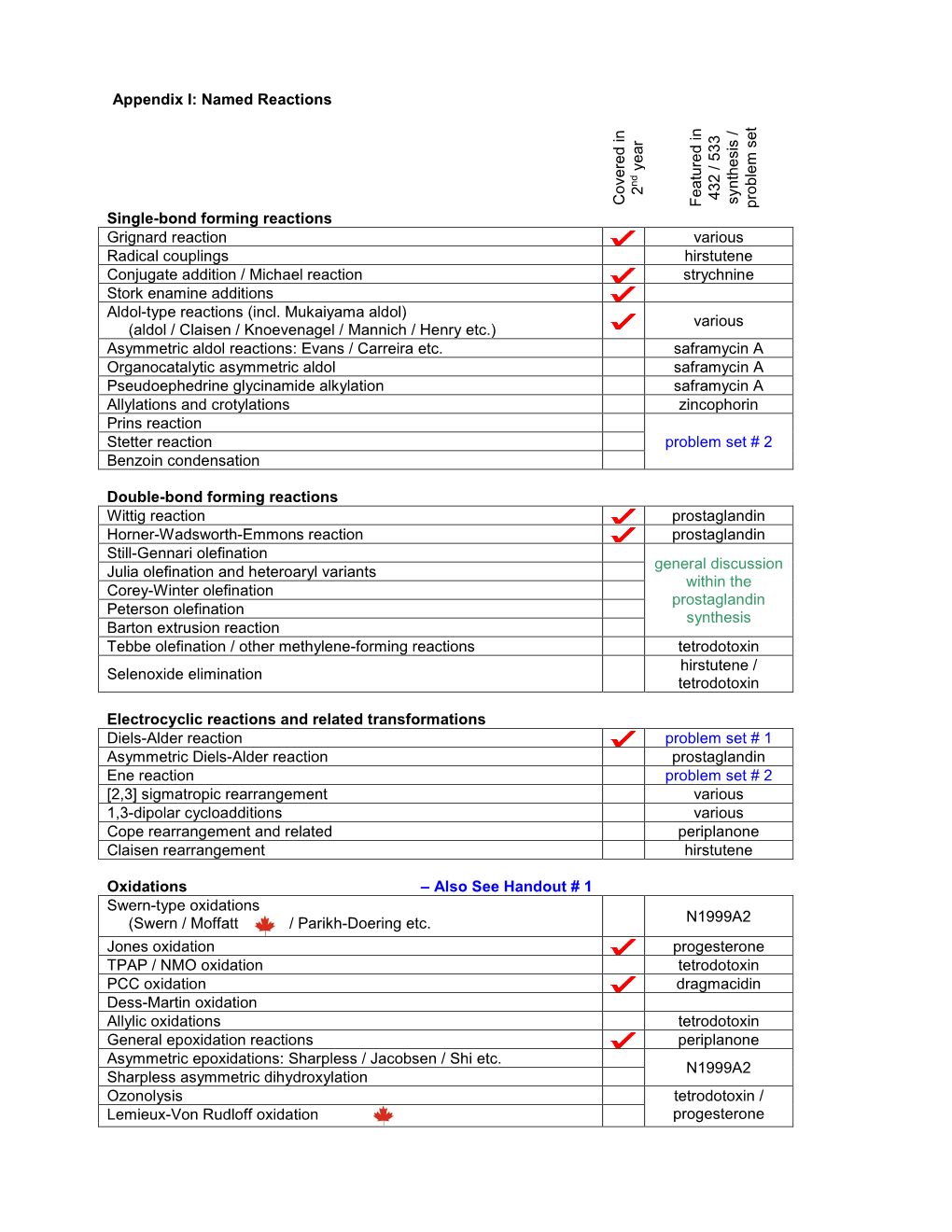
Appendix I: Named Reactions 235 / 335 432 / 533 synthesis / / synthesis Covered in Covered Featured in problem set problem Single-bond forming reactions Grignard reaction various Radical couplings hirstutene Conjugate addition / Michael reaction strychnine Stork enamine additions Aldol-type reactions (incl. Mukaiyama aldol) various (aldol / Claisen / Knoevenagel / Mannich / Henry etc.) Asymmetric aldol reactions: Evans / Carreira etc. saframycin A Organocatalytic asymmetric aldol saframycin A Pseudoephedrine glycinamide alkylation saframycin A Prins reaction Prins-pinacol reaction problem set # 2 Morita-Baylis-Hillman reaction McMurry condensation Gabriel synthesis problem set #3 Double-bond forming reactions Wittig reaction prostaglandin Horner-Wadsworth-Emmons reaction prostaglandin Still-Gennari olefination general discussion Julia olefination and heteroaryl variants within the Corey-Winter olefination prostaglandin Peterson olefination synthesis Barton extrusion reaction Tebbe olefination / other methylene-forming reactions tetrodotoxin hirstutene / Selenoxide elimination tetrodotoxin Burgess dehydration problem set # 3 Electrocyclic reactions and related transformations Diels-Alder reaction problem set # 1 Asymmetric Diels-Alder reaction prostaglandin Ene reaction problem set # 3 1,3-dipolar cycloadditions various [2,3] sigmatropic rearrangement various Cope rearrangement periplanone Claisen rearrangement hirstutene Oxidations – Also See Handout # 1 Swern-type oxidations (Swern / Moffatt / Parikh-Doering etc. N1999A2 Jones oxidation -

Steam Cracking: Chemical Engineering
Steam Cracking: Kinetics and Feed Characterisation João Pedro Vilhena de Freitas Moreira Thesis to obtain the Master of Science Degree in Chemical Engineering Supervisors: Professor Doctor Henrique Aníbal Santos de Matos Doctor Štepánˇ Špatenka Examination Committee Chairperson: Professor Doctor Carlos Manuel Faria de Barros Henriques Supervisor: Professor Doctor Henrique Aníbal Santos de Matos Member of the Committee: Specialist Engineer André Alexandre Bravo Ferreira Vilelas November 2015 ii The roots of education are bitter, but the fruit is sweet. – Aristotle All I am I owe to my mother. – George Washington iii iv Acknowledgments To begin with, my deepest thanks to Professor Carla Pinheiro, Professor Henrique Matos and Pro- fessor Costas Pantelides for allowing me to take this internship at Process Systems Enterprise Ltd., London, a seven-month truly worthy experience for both my professional and personal life which I will certainly never forget. I would also like to thank my PSE and IST supervisors, who help me to go through this final journey as a Chemical Engineering student. To Stˇ epˇ an´ and Sreekumar from PSE, thank you so much for your patience, for helping and encouraging me to always keep a positive attitude, even when harder problems arose. To Prof. Henrique who always showed availability to answer my questions and to meet in person whenever possible. Gostaria tambem´ de agradecer aos meus colegas de casa e de curso Andre,´ Frederico, Joana e Miguel, com quem partilhei casa. Foi uma experienciaˆ inesquec´ıvel que atravessamos´ juntos e cer- tamente que a vossa presenc¸a diaria´ apos´ cada dia de trabalho ajudou imenso a aliviar as saudades de casa. -

2 Results and Discussion
22 2 Results and Discussion 2 Results and Discussion 2.1 Syntheses of Azoninones - Unsaturated Nine-Membered Ring Lactams 2.1.1 Syntheses of Vinyl pyrrolidines N-Vinyl pyrrolidines are important reactants for the preparation of azoninones by zwitterionic aza- Claisen rearrangement. They can be obtained by derivatisation reactions starting from chiral molecules such as L-proline and 2S,4R-4-hydroxyproline or by metal catalysed cyclisation reactions of N- substituted allenic amines.66 Since large amounts of vinyl pyrrolidines have to be synthetically available for the systematic examination of the aza-Claisen rearrangement and its application in a total synthesis, especially the ex-chiral pool synthesis of these precursors has been extensively investigated by our group.67 The following two schemes represent the synthesis of the vinyl pyrrolidines [6] and [11] that have been used in this work as precursors for the aza-Claisen rearrangement. This synthesis allows the preparation of 50-100g of vinyl pyrrolidines. HO HO HO BnCl, Et3N TBSCl, imid. AcCl, MeOH CH Cl CH2Cl2 2 2 N COOH CO Me 100% N CO2Me 88% N 2 95% H H Bn [1] [2] [3] TBSO TBSO 1) Oxalyl chloride TBSO DMSO, Et3N, CH2Cl2 DIBALH, THF 2) Ph3P=CH2, THF N CO2Me N 89% N 70% Bn Bn OH Bn [4] [5] [6] Scheme 19 Synthesis of vinyl pyrrolidine [6] Allylamine [6] was efficiently generated via a six-step sequence starting from trans-4-hydroxy-L-(-)- proline [1] the overall yield was about 50% (Scheme 19). After esterification,68 the N-benzyl group 66 (a) Huby, N. -

Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters
Master in Chemical Engineering Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters Thesis of the Master’s Degree Development Project in Foreign Environment Cláudia Sofia Martins Angeira Erasmus coordinator: Eng. Miguel Madeira Examiner in FEUP: Eng. Fernando Martins Supervisor in ICT: Doc. Ing Petr Zámostný July 2008 Acknowledgements I would like to thank Doc. Ing. Petr Zámostný for the opportunity to hold a master's thesis in this project, the orientation, the support given during the laboratory work and suggestions for improvement through the work. i Hydrocarbons Thermal Cracking Selectivity Depending on Their Structure and Cracking Parameters Abstract This research deals with the study of hydrocarbon thermal cracking with the aim of producing ethylene, one of the most important raw materials in Chemical Industry. The main objective was the study of cracking reactions of hydrocarbons by means of measuring the selectivity of hydrocarbons primary cracking and evaluating the relationship between the structure and the behavior. This project constitutes one part of a bigger project involving the study of more than 30 hydrocarbons with broad structure variability. The work made in this particular project was focused on the study of the double bond position effect in linear unsaturated hydrocarbons. Laboratory experiments were carried out in the Laboratory of Gas and Pyrolysis Chromatography at the Department of Organic Technology, Institute of Chemical Technology, Prague, using for all experiments the same apparatus, Pyrolysis Gas Chromatograph, to increase the reliability and feasibility of results obtained. Linear octenes with different double bond position in hydrocarbon chain were used as model compounds. In order to achieve these goals, the primary cracking reactions were studied by the method of primary selectivities. -

Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes
Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes Stephen Goble Organic Super-Group Meeting Literature Presentation October 6, 2004 1 Outline I. Background • Introduction to Carbometallation • “Inner Sphere” vs. “Outer Sphere” • Review of Seminal Work II. Catalytic Palladium Systems III. Catalytic Platinum Systems IV. Catalytic Gold Systems 2 I. Background: Carbometallation is the formal addition of a metal and a carbon atom across a double bond. • Cis addition would involve olefin insertion in to an σ-alkyl-metal species. This is an “Inner Coordination Sphere” process. cis addition [M] [M] R olefin insertion R • The alkyl-metal species could arise from: 1. Oxidative addition of the metal to an electrophile. 2. Transmetallation of a nucleophile to the metal. 3. Direct nucleophilic attack on the palladium. • Trans addition would involve an “Outer Coordination Sphere” nucleophilic attack on the metal-olefin coordination complex. trans addition [M] [M] R- R 3 First Example: Carbopalladation of an Olefin Tsuji, J. and Takahashi, H. J. Am. Chem. Soc. 1965. 87(14), 3275-3276. [Pd] O O RO2C CO R PdCl2 RO OR 2 Na2CO3 [Pd] Cl • Amino and Oxo-palladations (i.e. Wacker Process) were previously known and have since been much more widely studied. • No distinction between cis and trans nucleophilic addition made. 4 Carbopalladation on Styrene: Different Modes of Attack Proposed different modes of attack based on different observed products with MeLi vs. Na-Malonate: Murahashi, S. et. al. J. Org. Chem. 1977. 42 (17), 2870-2874. Nucleophilic Attack on Pd Olefin Insertion B-Hydride Elimination CH3Li [Pd] [Pd] CH3 PdX2 CH3 [Pd]-H Inner Sphere Mechanism Palladium Coordination Nucleophilic Attack B-hydride elimination CO R CO2R 2 NaCH(CO2R) CO R [Pd] CO2R 2 PdX2 [Pd] [Pd]-H • Outer Sphere attack usually occurs at the more substituted carbon - more stabilization of + + charge (In intermolecular processes). -

Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals
Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Ho, Chun-Yu, Kristin Schleicher, Chun-Wa Chan, and Timothy Jamison. “Catalytic Addition of Simple Alkenes to Carbonyl Compounds by Use of Group 10 Metals.” Synlett 2009, no. 16 (October 4, 2009): 2565-2582. As Published http://dx.doi.org/10.1055/s-0029-1217747 Publisher Thieme Publishing Group Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/82119 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ NIH Public Access Author Manuscript Synlett. Author manuscript; available in PMC 2011 September 6. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Synlett. 2009 October 1; 2009(16): 2565±2582. doi:10.1055/s-0029-1217747. Catalytic Addition of Simple Alkenes to Carbonyl Compounds Using Group 10 Metals Chun-Yu Hoa, Kristin D. Schleicherb, and Timothy F. Jamisonb Chun-Yu Ho: [email protected]; Timothy F. Jamison: [email protected] a Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR (P.R. China), Fax: (852) 2603-5057 b Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA), Fax: (+1) 617-324-0253 Abstract Recent advances using nickel complexes in the activation of unactivated monosubstituted olefins for catalytic intermolecular carbon–carbon bond-forming reactions with carbonyl compounds, such as simple aldehydes, isocyanates, and conjugated aldehydes and ketones, are discussed. -

University of California
UC Riverside UC Riverside Electronic Theses and Dissertations Title Towards a Catalytic Asymmetric Cope Rearrangement and the Synthesis and Self-Assembly of Metal-Coordinated Hosts Permalink https://escholarship.org/uc/item/4gc654st Author Moehlig, Melissa Padilla Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Towards a Catalytic Asymmetric Cope Rearrangement and the Synthesis and Self- Assembly of Metal-Coordinated Hosts A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry by Melissa Padilla Moehlig December 2013 Dissertation Committee: Dr. Richard J. Hooley, Chairperson Dr. Catharine H. Larsen Dr. Michael C. Pirrung Copyright by Melissa Padilla Moehlig 2013 The Dissertation of Melissa Padilla Moehlig is approved: Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS Graduate school has been one of the most rewarding and yet the most exhausting and stressful times of my life. It would not have survived without the help of several people. I would like to thank Dr. Courtney Meyet, Dr. Katherine Djernes, and Yoo-Jin Ghang for their friendship and all the laughs that were necessary to keep me sane. I would like to thank Michael Young, Hou Ung, and Jay-Ar Bendo for our morning coffee breaks, they were crucial to get my day started. I would like to thank Prof. Larsen for teaching me to be independent. I would like to thank Prof. Hooley for all his guidance over the past five years. You are truly a great mentor and I don’t think I would have survived graduate school without your help and advice. -

© Cambridge University Press Cambridge
Cambridge University Press 0521770971 - Modern Methods of Organic Synthesis W. Carruthers and Iain Coldham Index More information Index acidity 1 from alkynes 125–32 acyl anion equivalents 56 from diols 123 acyloin reaction 425 from hydrazones 120 Adams’ catalyst 407 reaction of AD-mix ␣ 352 with carbenes 303–9 AD-mix  352 with dienes in Diels–Alder reaction 162 agelastatin A 376 with radicals 280–98 AIBN 268 reduction of 322, 408–13, 459 alane 437, 444 alkenyllithium species 57, 59 alcohols alkylation 1–19 deoxygenation 270 asymmetric 37 from alkenes 323, 349 with enamines 1, 17 from carbonyl compounds 416, 421, 423, 434–56 with enolates 1–16 oxidation 378–93 with metalloenamines 16 aldehydes alkyl halides alkylation of 17 oxidation to carbonyl compounds 384 as dienophiles in Diels–Alder reaction 169 reductive cleavage to hydrocarbons 269, 406, 442 decarbonylation of 419 alkyllithium species 46 from alcohols 380 alkynes from alkenes 325, 360, 364 conversion to alkenes 125–32, 414 oxidation of 392 deprotonation of 58 reduction of 435, 439, 443 hydrometallation 128 reductive dimerization of 148, 425 preparation of 137 Alder–ene reaction 231 reduction of 125 aldol reaction 27–36 allopumiliotoxin 58 diastereoselective 32 allosamidin disaccharides 272 enantioselective 41 allylic organometallics 71–4 aldosterone 276 allylic oxidation 374 alkenes allylic 1,3-strain 26, 73, 351 allylic oxidation of 374 -allylpalladium complexes 98 conversion to alcohols 323 amabiline 220 conversion to ketones (Wacker reaction) 365 ambruticin S 308 epoxidation -

Named Organic Reactions
Named Organic Reactions Thomas Laue and Andreas Piagens Technical University, Braunschweig, Germany Translated into English by Dr. Claus Vogel Universität Magdeburg, Germany JOHN WILEY & SONS Chichester • New York • Weinheim • Brisbane • Singapore • Toronto Contents Introduction ix Acyloin Ester Condensation 1 Aldol Reaction 4 Alkene Metathesis 10 Arbuzov Reaction 12 Arndt-Eistert Synthesis 13 Baeyer-Villiger Oxidation 16 Bamford-Stevens Reaction 19 Beckmann Rearrangement 22 Benzidine Rearrangement 24 Benzilic Acid Rearrangement 26 Benzoin Condensation 27 Bergman Cyclization 29 Birch Reduction 33 Blanc Reaction 36 Bucherer Reaction 37 Cannizzaro Reaction 40 Chugaev Reaction 42 Claisen Ester Condensation 45 Claisen Rearrangement 48 Clemmensen Reduction 52 Cope Elimination Reaction 54 Cope Rearrangement 56 Corey-Winter Fragmentation 59 Curtius Reaction 61 1,3-Dipolar Cycloaddition 64 [2 + 2] Cycloaddition 67 Darzens Glycidic Ester Condensation 71 Delepine Reaction 73 Diazo Coupling 74 Diazotization 77 Diels-Alder Reaction 78 vi Contents Di-7r-Methane Rearrangement 86 Dötz Reaction 88 Elbs Reaction 92 Ene Reaction 93 Ester Pyrolysis 97 Favorskii Rearrangement 100 Finkelstein Reaction 102 Fischer Indole Synthesis 103 Friedel-Crafts Acylation 106 Friedel-Crafts Alkylation 110 Friedländer Quinoline Synthesis 114 Fries Rearrangement 116 Gabriel Synthesis 120 Gattermann Synthesis 123 Glaser Coupling Reaction 125 Glycol Cleavage 127 Gomberg-B achmann Reaction 129 Grignard Reaction 132 Haloform Reaction 139 Hantzsch Pyridine Synthesis 141 Heck Reaction -

C9sc03756j1.Pdf
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information for Orthogonal Functionalization of Alternating Polyesters: Selective Patterning of (AB)n Sequences Ni Yi,a† Thomas T. D. Chen,a† Junjuda Unruangsri,b Yunqing Zhu,b and Charlotte K. Williamsa* a Chemistry researCh laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK b Department of Chemistry, Imperial College London, London, SW7 2AZ, UK †Joint first authors [email protected] TaBle of Contents SeCtion, Scheme, Table or Figure Pages Experimental SeCtion 3-6 Figure S1. StruCtures of the alternating polyesters P1-P9, all featuring internal and 4 terminal alkene funCtionalities. TaBle S1. Composition and Properties of P1-P9. 5 Scheme S1. One-pot hydroboration-oxidation reaCtion to Convert the polyester 5 terminal alkenes to alternating hydroxyl groups. Scheme S2. Photo-initiated thiol-ene reaCtions to transform the hydroxyl- 6 funCtionalized alternating polyesters into orthogonally funCtionalized (AB)n Polyesters TaBle S2. Preparation Data for Polymers P1(B) - P1(f) and data to aCCompany in Table 6 1. Figure S2. NMR speCtra for P1. 7 Figure S3. 2D NMR speCtra for P1. 8 1 Figure S4. H NMR speCtrum of polymer P1 (CDCl3, 500.0 MHz, 298 K). 9 13 1 Figure S5. C{ H} NMR speCtrum of P1 (d6-DMSO, 500.0 MHz, 298 K). 9 Figure S6. MALDI-ToF speCtrum of P1 synthesized with CHD as the CTA, (Table S1, #1). 10 TaBle S3. SEC CharaCterization data (in THF and DMF) for P1, P2 and P3. -

PDF (Appendix 2: the Cope Rearrangement)
65 APPENDIX TWO The Cope Rearrangement A2.1 The Cope Rearrangement In 1940, Arthur Cope discovered a thermal rearrangement of 1,5-diene 137a to a more conjugated isomeric 1,5-diene (137b, Scheme A2.1.1).1 Cope postulated that his rearrangement was an all-carbon analogue of the Claisen rearrangement,2 and was intramolecular. He speculated that the reaction proceeded through a six-membered transition state. Cope published these hypotheses 25 years before Woodward and Hoffman3 disclosed the first papers on conservation of orbital symmetry, a theory that explained the molecular orbital basis for synchronous Cope rearrangements, calling them [3,3] sigmatropic rearrangements. Scheme A2.1.1 The Cope rearrangement 150–160 °C NC NC Me 4 h Me EtO2C EtO2C Me Me 137a 137b A2.2 Transition State Geometry in Concerted Cope Rearrangements4 Thermal [3,3] sigmatropic rearrangements occur with suprafacial–suprafacial geometries3 through a six-membered cyclic transition state in either a chair or boat conformation. In 1962, Doering and Roth5 determined that in simple cases, the Cope rearrangement proceeded through a chair-like transition state (Scheme A2.2.1). On the basis of product ratios, Doering and Roth estimated that ΔΔG‡ (boat–chair) for meso 66 138a was 5.7 kcal/mol. Subsequent experiments by Hill excluded the twist (helix) arrangement,6 which had not been considered by Doering and Roth. Scheme A2.2.1 Feasible transition states for the Cope rearrangement Me Me Me Me ! Me Me Me Me (99.7% yield) 138b Me Me Me Me ! ! Me Me Me Me Me Me (0.3% yield) 138a 138c Me Me Me Me ! Me Me Me Me 138d While simple Cope rearrangements employ a chair transition state, the boat transition state is used when molecules are geometrically constrained, as is the case with 1,2-divinyl cyclopropanes in which the vinyl groups are nearly elipsed.7 Most 1,2- divinylcyclobutanes also proceed through boat transition states,8,9 but their larger ring size accompanies greater structural flexibility.