Geochemical and Boron, Strontium, and Oxygen Isotopicconstraints On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Deficits and Dependence to Balanced Budgets and Independence
From Deficits and Dependence to Balanced Budgets and Independence The Arab Local Authorities’ Revenue Sources Michal Belikoff and Safa Agbaria Edited by Shirley Racah Jerusalem – Haifa – Nazareth April 2014 From Deficits and Dependence to Balanced Budgets and Independence The Arab Local Authorities’ Revenue Sources Michal Belikoff and Safa Agbaria Edited by Shirley Racah Jerusalem – Haifa – Nazareth April 2014 From Deficits and Dependence to Balanced Budgets and Independence The Arab Local Authorities’ Revenue Sources Research and writing: Michal Belikoff and Safa Ali Agbaria Editing: Shirley Racah Steering committee: Samah Elkhatib-Ayoub, Ron Gerlitz, Azar Dakwar, Mohammed Khaliliye, Abed Kanaaneh, Jabir Asaqla, Ghaida Rinawie Zoabi, and Shirley Racah Critical review and assistance with research and writing: Ron Gerlitz and Shirley Racah Academic advisor: Dr. Nahum Ben-Elia Co-directors of Sikkuy’s Equality Policy Department: Abed Kanaaneh and Shirley Racah Project director for Injaz: Mohammed Khaliliye Hebrew language editing: Naomi Glick-Ozrad Production: Michal Belikoff English: IBRT Jerusalem Graphic design: Michal Schreiber Printed by: Defus Tira This pamphlet has also been published in Arabic and Hebrew and is available online at www.sikkuy.org.il and http://injaz.org.il Published with the generous assistance of: The European Union This publication has been produced with the assistance of the European Union. Its contents are the sole responsibility of Sikkuy and Injaz and can in no way be taken to reflect the views of the European Union. The Moriah Fund UJA-Federation of New York The Jewish Federations of North America Social Venture Fund for Jewish-Arab Equality and Shared Society The Alan B. -

The National Left (First Draft) by Shmuel Hasfari and Eldad Yaniv
The National Left (First Draft) by Shmu'el Hasfari and Eldad Yaniv Open Source Center OSC Summary: A self-published book by Israeli playwright Shmu'el Hasfari and political activist Eldad Yaniv entitled "The National Left (First Draft)" bemoans the death of Israel's political left. http://www.fas.org/irp/dni/osc/israel-left.pdf Statement by the Authors The contents of this publication are the responsibility of the authors, who also personally bore the modest printing costs. Any part of the material in this book may be photocopied and recorded. It is recommended that it should be kept in a data-storage system, transmitted, or recorded in any form or by any electronic, optical, mechanical means, or otherwise. Any form of commercial use of the material in this book is permitted without the explicit written permission of the authors. 1. The Left The Left died the day the Six-Day War ended. With the dawn of the Israeli empire, the Left's sun sank and the Small [pun on Smol, the Hebrew word for Left] was born. The Small is a mark of Cain, a disparaging term for a collaborator, a lover of Arabs, a hater of Israel, a Jew who turns against his own people, not a patriot. The Small-ists eat pork on Yom Kippur, gobble shrimps during the week, drink espresso whenever possible, and are homos, kapos, artsy-fartsy snobs, and what not. Until 1967, the Left actually managed some impressive deeds -- it took control of the land, ploughed, sowed, harvested, founded the state, built the army, built its industry from scratch, fought Arabs, settled the land, built the nuclear reactor, brought millions of Jews here and absorbed them, and set up kibbutzim, moshavim, and agriculture. -

Return of Organization Exempt from Income
Return of Organization Exempt From Income Tax Form 990 Under section 501 (c), 527, or 4947( a)(1) of the Internal Revenue Code (except black lung benefit trust or private foundation) 2005 Department of the Treasury Internal Revenue Service ► The o rganization may have to use a copy of this return to satisfy state re porting requirements. A For the 2005 calendar year , or tax year be and B Check If C Name of organization D Employer Identification number applicable Please use IRS change ta Qachange RICA IS RAEL CULTURAL FOUNDATION 13-1664048 E; a11gne ^ci See Number and street (or P 0. box if mail is not delivered to street address) Room/suite E Telephone number 0jretum specific 1 EAST 42ND STREET 1400 212-557-1600 Instruo retum uons City or town , state or country, and ZIP + 4 F nocounwro memos 0 Cash [X ,camel ded On° EW YORK , NY 10017 (sped ► [l^PP°ca"on pending • Section 501 (Il)c 3 organizations and 4947(a)(1) nonexempt charitable trusts H and I are not applicable to section 527 organizations. must attach a completed Schedule A ( Form 990 or 990-EZ). H(a) Is this a group return for affiliates ? Yes OX No G Website : : / /AICF . WEBNET . ORG/ H(b) If 'Yes ,* enter number of affiliates' N/A J Organization type (deckonIyone) ► [ 501(c) ( 3 ) I (insert no ) ] 4947(a)(1) or L] 527 H(c) Are all affiliates included ? N/A Yes E__1 No Is(ITthis , attach a list) K Check here Q the organization' s gross receipts are normally not The 110- if more than $25 ,000 . -
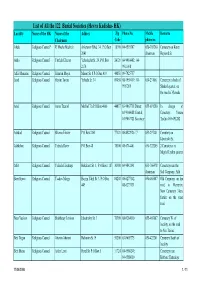
List of All the 122 Burial Societies (Hevra Kadisha- HK) Locality Name of the HK Name of the Addres Zip Phone No
List of All the 122 Burial Societies (Hevra Kadisha- HK) Locality Name of the HK Name of the Addres Zip Phone No. Mobile Remarks Chairman Code phone no. Afula Religious Council* R' Moshe Mashiah Arlozorov Blvd. 34, P.O.Box 18100 04-6593507 050-303260 Cemetery on Keren 2041 chairman Hayesod St. Akko Religious Council Yitzhak Elharar Yehoshafat St. 29, P.O.Box 24121 04-9910402; 04- 2174 9911098 Alfei Menashe Religious Council Shim'on Moyal Manor St. 8 P.O.Box 419 44851 09-7925757 Arad Religious Council Hayim Tovim Yehuda St. 34 89058 08-9959419; 08- 050-231061 Cemetery in back of 9957269 Shaked quarter, on the road to Massada Ariel Religious Council Amos Tzuriel Mish'ol 7/a P.O.Box 4066 44837 03-9067718 Direct; 055-691280 In charge of 03-9366088 Central; Cemetery: Yoram 03-9067721 Secretary Tzefira 055-691282 Ashdod Religious Council Shlomo Eliezer P.O.Box 2161 77121 08-8522926 / 7 053-297401 Cemetery on Jabotinski St. Ashkelon Religious Council Yehuda Raviv P.O.Box 48 78100 08-6714401 050-322205 2 Cemeteries in Migdal Tzafon quarter Atlit Religious Council Yehuda Elmakays Hakalanit St. 1, P.O.Box 1187 30300 04-9842141 053-766478 Cemetery near the chairman Salt Company, Atlit Beer Sheva Religious Council Yaakov Margy Hayim Yahil St. 3, P.O.Box 84208 08-6277142, 050-465887 Old Cemetery on the 449 08-6273131 road to Harzerim; New Cemetery 3 km. further on the same road Beer Yaakov Religious Council Shabbetay Levison Jabotinsky St. 3 70300 08-9284010 055-465887 Cemetery W. -

רשימת קידוחי נפט וגז טבעי בישראל Index of Oil and Gas Wells in Israel Updated to 18.11.2017 No
Page 1 of 5 רשימת קידוחי נפט וגז טבעי בישראל Index of Oil and Gas Wells in Israel updated to 18.11.2017 No. WellNo Name Company East North SpudDate CompDate AgeTD TD KB GL RT WD 1 392 ADMON 01 INOC Dead Sea Ltd. Partn. 236526 549741 07/09/1990 01/11/1990 PLEISTOCENE 2179 -378.4 -388.16 2 81 AFIQ 01 Lapidoth Israel Oil Prospectors Corp. Ltd. 153386 594304 21/08/1959 24/12/1959 EARLY CRETACEOUS 2002 90 86.6 89.5 3 303 AFIQ 02 OEL 151649 599226 10/11/1980 09/12/1980 EARLY CRETACEOUS 1577 58.82 54.15 58.37 4 380 AGUR 01 Emog 142141 550678 10/02/1987 27/07/1987 LIAS (EARLY JURASSIC) 3922 181.3 171.45 180.95 5 380A AGUR 01A DEEP Avner Oil and Gas 142141 550678 18/09/1992 03/01/1993 PALEOZOIC 5147 181.3 171.45 180.95 6 313 AMAZYAHU 01 OEL 236981 540907 19/11/1981 20/02/1982 PLEISTOCENE 2500 -368.21 -372.23 -368.63 7 318 AMAZYAHU 01A OEL 236972 540948 11/05/1982 14/05/1982 PLEISTOCENE 201 -370.4 -372.51 8 319 AMAZYAHU 02 OEL 237717 540480 17/05/1982 22/05/1982 PLEISTOCENE 186 -369.95 -372 9 320 AMAZYAHU 03 OEL 236515 538422 25/05/1982 02/06/1982 PLEISTOCENE 207 -352.27 -354.27 10 321 AMAZYAHU 04 OEL 235955 540333 05/06/1982 08/06/1982 PLEISTOCENE 220 -367.37 -369.34 11 323 AMAZYAHU 05 OEL 239605 547143 18/06/1982 23/06/1982 PLEISTOCENE 204 -383.2 -385.23 12 322 AMAZYAHU 06 OEL 237708 544016 12/06/1982 14/06/1982 PLEISTOCENE 201 -380.7 -382.7 13 256 AMIAZ 01 OEL 234837 555616 03/03/1976 30/09/1976 DOGGER (MID JURASSIC) 4613 -263.88 -268.18 -264.38 14 393 AMIAZ EAST 01 INOC Dead Sea Ltd. -

I Name Postal Address and Number of Telephone Giv'at Hen Giv'at
I xn Postal Address and Number of Telephone Name Postal Address and Number oi Telephone Name RaanannaPO Tel 921122 Raananna Ibtin Kefar Hasidim PO Tel 41 Kefar Hasidim Giv'at Hen Ha-Merkaz Mobile PO Tel 971117 Lod Airport Iddit Rehovot POB 547 Giv'at Koah Nes Ziyyona PO Tel 943209 Nes Ziyyona Iksal Iksal PO Tel 4339 Nazareth Giv'at Mikhae Binyamina PO Tel 8034 Binyamina Ilaniyya Ha-Galil Ha-Tahton Mobile PO Giv'at Nili Hevel Megiddo Mobile PO Tel 7221 Kefar Tavor Giv'at Noah Ilanot Lev Ha-Sharon Mobile PO Tel 2530 Netanya Giv'at Oz Hevel Megiddo Mobile PO Tel 2422 Afula Avihayil PO Tel 3583 Netanya Hut Nazareth PO Giv'at Shappira Isfiya Tsfiya PO Tel (see Haifa Exchange) Giv'at Shemuel Giv'at Shemuel PO Tel 722531 Ramat Gan Giv'at Yearim Hare Yehuda Mobile PO Tel 28597 Jerusalem Giv'at Yesha'yahu Ha-Ela Mobile PO Tel 262 Bet Shemesh Giv'atayim PO Tel (see TA-Yafo Section) Giv'atayim Jaljuliya Ha-Merkaz Mobile PO Giv'ati Evtah Mobile PO Tel 954128 Qiryat Mal'akhi Ha-Negev Mobile PO Tel 230 Netivot Tel 924193 Hadar Ramatayim Giv'olim Jati Shomrom Mobile PO Tel 7090 Pardess Hanna Gonen Ha-Galil Ha-Elyon Mobile PO Tel 48023, 48033 Neot Mordekhay Jatt Maale Ha-Galil Mobile PO Ha-Galil Ha-Maaravi Mobile PO Jerusalem Tel (see Jerusalem Exchange; Goren Jisr az Zarqa Tel 926032 Shelomi Binyamina PO Tel 8198 Binyamina Judeida Merom Ha-Galil Mobile PO Maale Ha-Galil Mobile PO Gush Halav Julis Maale Ha-Galil Mobile PO Jurdeih Shelomi PO H Ha-Bonim Hof Karmel Mobile PO Tel 942034 Atlit Hadar Am Lev Ha-Sharon Mobile PO Tel 3493 Netanya K Hadar Ramatayim -

Back and Forth: Commuting for Work in Israel Haim Bleikh*
1 Executive Summary Back and Forth: Commuting for Work in Israel Haim Bleikh* Full research study published in October 2018 The subject of commuting has attracted more and more public attention in Israel in recent years as road congestion levels continue to rise with the increasing number of commuters. Over the last 30 years, the number of employed persons working outside their residential area has risen from 42 percent to 54 percent (as of 2016) among Israelis of working age (25-64). The main mode of commuting is by private car and the number of rides has grown faster than road expansion — creating the traffic jams that have become all too familiar. Commuting distance, time, and mode of transportation Most trips to work are short. Three out of every four workers ages 25-64 travel 20 kilometers or less to reach their workplace, mostly in private vehicles (for 2014-2016). About 60 percent of workers travel for no more than half an hour, 30 percent between half an hour and an hour, and about 10 percent travel for over an hour in each direction. Regarding the choice in mode of transportation — 62 percent commute to work by car (including shared rides) and only 17 percent commute by public transportation. About 10 percent commute by bicycle or by foot and 8 percent commute by work- organized transportation. There are large differences in commuting patterns in different parts of the country. For example, in both Jerusalem and Petah Tikva many commuters travel between half an hour and an hour, but in Jerusalem (where 91 percent of residents work within the city) this seems to be due to the extensive use of public transportation and large city size while, in Petah Tikva, a higher percentage use a private vehicle and commute distances of up to 20 kilometers, indicating that the travel time is a result of traffic congestion. -

GROUND-WATER POLLUTION DETERMINED by XA9848325 BORON ISOTOPE SYSTEMATICS A. VENGOSH Research Department, Hydrological Service, J
GROUND-WATER POLLUTION DETERMINED BY XA9848325 BORON ISOTOPE SYSTEMATICS A. VENGOSH Research Department, Hydrological Service, Jerusalem, Israel Y. KOLODNY Institute of Earth Sciences, Hebrew University, Giva't Ram, Jerusalem, Israel A.J. SPIVACK Center for Marine Sciences, University of North Carolina at Wilmington, Wilmington, United States of America Abstract-Soro/? isotopic systematics as related to ground-water pollution is reviewed. We report isotopic results of contaminated ground water from the coastal aquifers of the Mediterranean in Israel, Cornia River in north-western Italy, and Salinas Valley, California. In addition, the B isotopic composition of synthetic B compounds used for detergents and fertilizers was investigated. Isotopic analyses were carried out by negative thermal ionization mass spectrometry. The investigated ground water revealed different contamination sources; underlying saline water of a marine origin in saline plumes in the Mediterranean coastal aquifer of Israel (3]jB=31.7%o to 49.9%o, B/Cl ratio-UxlO'3), mixing of fresh and sea water (25%o to 38%o, B/Cl~7xJ0~3) in saline water associated with salt-water intrusion to Salinas Valley, California, and a hydrothermal contribution (high B/Cl of -0.03, SIIB=2.4%o to 9.3%o) in ground water from Cornia River, Italy. The&'B values of synthetic Na-borate products (-0.4%o to 7.5%o) overlap with those of natural Na-borate minerals (-0.9%o to 10.2%o). In contrast, the S^B values of synthetic Ca-borate and Na/Ca borate products are significantly lower (-15%o to -12.1%o) and overlap with those of the natural Ca-borate minerals. -

New Crypto Currency Mining Malware Also Disables Security Services
MAY 2018 New Crypto Currency Mining Malware Also Disables Security Services During April 2018, Fortinet Cyber Security Company discovered a new cryptocurrency miner named Monero that not only allows attackers to use the infected computer to mine the cryptocurrency but also disables the cybersecurity services. Monero or PyroMiner is downloaded as a zip, and once inside, it takes control of the Remote Desktop Protocol, a protocol that allows a computer to connect with another one over a network connection, and modify the firewall to allow the malware to get through. The attackers can now block Windows Update, disable the rest of the cybersecurity defenses, and keep installing malware on the victim’s computer. So far, PyroMiner only attacks Windows-powered computers, and it spreads using the EternalRomance exploit, a flaw in Windows coding patched by Microsoft in March 2017. Pinkerton considers likely that the malware will spread faster in the following months due to the number of users who have not updated their Windows operating system. On February 4, 2018, Tripwire Cyber Security Company published an article stating that EternalRomance, EternalSinergy, and EternalChampion exploits had been updated and now could efficiently spread through Windows 2000 to 2016 operative systems. Pinkerton advises all clients to update their antivirus database and Windows operative system. Additionally, it is recommended to avoid entering untrusted web pages and remain attentive to Windows announcements regarding software patches and updates. LinkedIn Security Liability May Have Compromised Users’ Personal Data A security researcher from Lightning Security has reported that he discovered a security bug which made it possible for third parties to access users’ data, including private information such as email addresses and phone numbers. -

The Egert – Cohen Guide to Medical Insurance in Israel
The Egert – Cohen guide to Medical Insurance in Israel Welcome! You are insured through Harel, Israel's premium health insurance company. Your individual policy number has been sent to you by e-mail. How to use the policy? 1) General Attached, you will find a list of doctors and medical services contracted to Harel – always confirm that the clinic is open before you arrive and mention that you are insured with Harel VIP. Also, use our apps to find all Medical Services – Download now!!! Android IOS * To use any of these services, just give your Harel policy # or passport # and you will be treated without any payment. There is no deductible or co-payment for medical services. 2) Hospital Emergency Rooms: Major emergencies (broken limb, loss of consciousness, poisoning, serious eye injury) – go directly to the EMR. In non-emergency situations, you must be referred by a Harel doctor/clinic, or through the on-line medical service. Telephone: 1800-260-660 or 03-6272300 ext. 1/3. * For other minor emergencies, go directly to the Terem 24 clinics – (see attached). * In emergency situation only, call an ambulance (102 in Israel). 3) Bikur Rofeh- You can arrange for a doctor home visit but remember: a) It can take longer than expected because of back-ups with other patients / groups. b) The doctors are not always of the highest level! Telephone: *6101 4) Prescription medication – there is no cash out outlay for pharmacies on the Harel list. If you are far from a registered pharmacy, you should pay for the medication and return both the prescription and the receipts to our office for a refund. -

List of Places in Israel
X LIST OF PLACES IN ISRAEL Postal Address and Number of Telephone Name Postal Address and Number of Telephone Name Bar'am Merom Ha-Galr Mobile PO Tel 30363 Zefat Baraq Gilboa Mobile PO Tel 2610 Afula Barekei Ha-Merkaz Mobile PO Tel 971056 Lod Airport Barqay Shomron Mobile PO Tel 7106 Pardess Hanna Abba Hillel Nahal Lakhish Zafon Mobile PO Tel 2248 Ashqelon Barta'a 'Ara PO Bat Shelomo Hof Karmel Mobile PO Tel 9113 Zikhron Yaakov Abu Ghosh Hare Yehuda Mobile PO Tel 28448 Jerusalem (Police) Bat Yam Bat Yam PO Tel (see TA-Yafo Exchange) Beer Ora Abu Sinan Kafr Yasii PO Elat PO Beer Sheva Adderet Ha-Ela Mobile PO Tel 256 Bet Shemesh Beer Sheva PO Tel (see Beer Sheva Exchange) Admit Nahariyya PO Tel 926043 Shelomi Beer Tuveya Beer Tuveya PO Tel (see Qiryat Malakhi Exchange) Adanim Hadar-Ramatayim PO Tel 911396 Petah Tiqwa Beer Yaakov Beer Yaakov PO Tel (see Ramla Exchange) Addirim Gilboa Mobile PO Tel 2464 Afula Beeri Ha-Negev Mobile PO Afeq AfeqPO Tel 71260 Haifa Tel 281 Ofaqim, 207 Saad Afiqim Afiqim PO Tel 50172 Kinneret Beerot Yitzhak Tel Aviv-Yafo PO Tel 911737 Petah Tiqwa Afula Afula PO Tel (see Afula Exchange) Beerotayim Lev Ha-Sharon Mobile PO Tel 8032 Kefar Yona Agur Ha-Ela Mobile PO Tel 214 Bet Shemesh Beit Yann Beit Yann PO Ahiezer LodPO Tel 962217 Lod Beit Jimal Jerusalem POB 159 Ahihud Asherat Mobile PO Tel 910114 Akko Bekhora Emeq Bet Shean Mobile PO Ahisamakb Ha-Merkaz Mobile PO Tel 962239 Lod Ben Ammi Asherat Mobile PO Tel 920354 Nahariyya Ahituv Shomron Mobile PO Tel 2308 Hadera Ben Shemen Ben Shemen PO Tel 962143 Lod Ahuzzam -

Alestine (3A3ette Jpublisbeb Bt Hutborits
XLhc alestine (3a3ette Jpublisbeb bt Hutborits No. 553 THURSDAY, 28TH NOVEMBER, 1935 1137 CONTENTS Page ORDINANCE CONFIRMED Confirmation of Ordinance, No. 41 of 1935 - 1138 GOVERNMENT NOTICES Appointments, etc. 1138 Notice regarding applications for Iraqi passports and visas - 1138 Notice regarding Telephone Service between Palestine and Iraq - 1139 Medical Licence cancelled - - - - - 1139 Claims for Destroyed Currency Notes - ' - - - 1139 ־ - - - Teachers' Higher Certificate Examination, 1935 1139 B. Sc. Economics Examination of the University of London, Jerusalem Centre 1140 Tender and Adjudication of Contracts ' 1140 Citation Orders !!44 RETURNS Persons entering and leaving Palestine during September, 1935 1142 ־ Persons Changing their Names . 1143 Quarantine and Infectious Diseases Summary .... !447 REGISTRATION OF COMPANIES, COOPERATIVE SOCIETIES, PARTNERSHIPS, ETC. 1147 REGISTRATION OF TRADE MARKS, PATENTS AND DESIGNS ... 1153 SUPPLEMENT No. 2. The following subsidiary legislation is published in Supplement No. 2 which forms part of this Gazette :— Hebron Municipal Council (Meetings and Proceedings) Regulations, 1935. under the Municipal Corporations Ordinance, 1934 1173 Jaffa (Entertainment Fees) RyIaws, 1935, under the Municipal Corporation Ordinance, 1934 1174 Lydda Municipal Bylaws, 1935, under the Municipal Corporations Ordinance, 1934 1179 Road Transport (Boutes and Tariffs) (A mendment No. 2) Rules, 1935, under the Road ־ Transport Ordinance, 1929 1189 Animal Quarantine (Poultry) Rules,