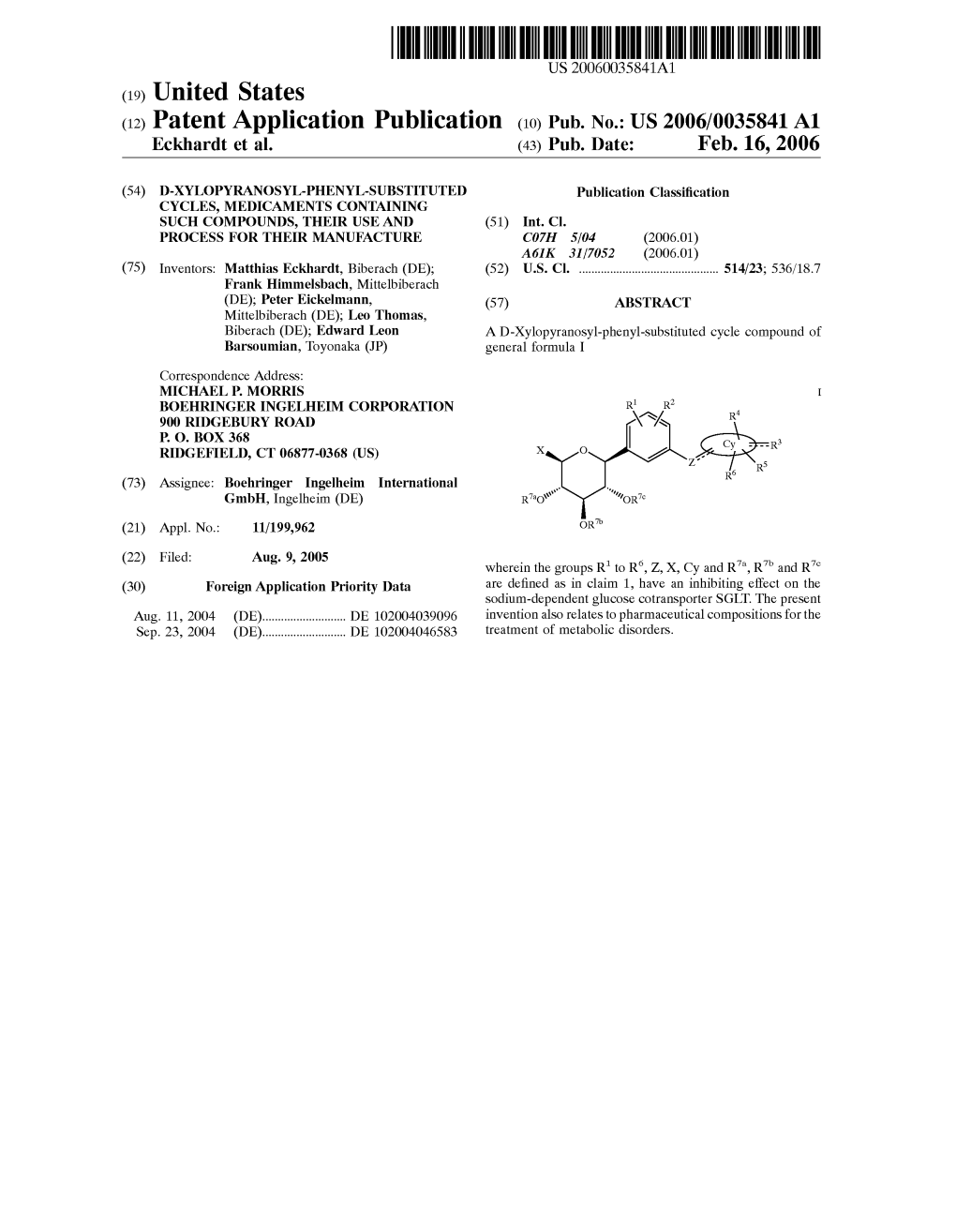
(12) Patent Application Publication (10) Pub. No.: US 2006/0035841A1 Eckhardt Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Reactive Methylene Compounds As Synthons for Various Bio Active
Reactive methylene compounds as synthons for various bio active molecules Mohd. Shahnawaz khan1*, Saba Parveen Siddiqui2, Daya S. Seth3 1Department of Chemistry, JK. Lakshmipat University Jaipur (Raj) -302026, India 2Department of Chemistry Kendriya Vidhayalaya N0-1 Pratap Nagar, Udaipur (Raj) 313001, India 3Department of Chemistry, School of Chemical Sciences, St. John's College, Agra (UP)-282002, India *E-mail: [email protected] Abstract: Novel malonamic acid, hydrazide and amide were efficiently synthesized from the condensation of 3-NO2 aniline with diethyl malonate. Also the synthesis of coumarins, azo coumarins. benzocoumarins, cinnamamide, and α:β-unsaturated acid, were achieved by the reaction of above synthesized compounds in a single step reaction in good to excellent yields. And these eight compounds were tested for their antibacterial activities with two bacteria E. coli and S. aureus. Compounds are showing slightly to moderate antibacterial activities against same bacterias. Key words: Reactive methylene compounds, antibacterial activity. Introduction: Organic compounds containing the reactive methylene group provide excellent intermediates in synthetic organic chemistry. Such substances have been found to be useful as synthons for various bioactive agents. Using such type of compounds as starting material quiet a large number of heterocyclic and non-heterocyclic compounds can be prepared by condensing them with other substances. Heterocycles form by far the largest of classical divisions of organic chemistry. A broad spectrum of biological activity associated with heterocyclic compounds has attracted interest in drug discovery research. As evident from literature, both synthetic as well as natural oxygen and nitrogen containing heterocyclic molecules possesses significant antimicrobial activities and a large number have been made up to clinics for health care worldwide. -

United States Patent (19) 11 Patent Number: 4,880,935 Thorpe 45 Date of Patent: Nov
United States Patent (19) 11 Patent Number: 4,880,935 Thorpe 45 Date of Patent: Nov. 14, 1989 (54. HETEROBIFUNCTIONAL LINKING Wang et al., Israel Journal of Chem., vol. 12, 1974, pp. AGENTS DERVED FROM 375-389. N-SUCCNMDO-DTHO-ALPHIA Vallero et al., Science, 222, 1983, pp. 512-515. METHYL-METHYLENE-BENZOATES Camber et al., Method of Enzymol, 112, 1985, pp. 201-225. 75 Inventor: Philip E. Thorpe, London, England Langone et al., Method of Enzymol, 93, 1983, p. 280. Masuho et al., J. Biochem, 91, 1982, pp. 1583-1591. 73 Assignee: ICRF (Patents) Limited, London, Carlsson et al., Biochem. J., 1978, 173, pp. 723-737. England Calombatti et al., J. Immunol, 131, 1983, pp. 3091-3095. Brown et al., Cancer Res., vol. 45, 1985, pp. 1214-1221. 21 Appl. No.: 90,386 Ramakrishnonet al., Cancer Res., 44, 1984, pp. 201-208. 22 Filed: Aug. 27, 1987 Gras et al., J. of Immunol Methods, 81, 1985, pp. 283-297. Related U.S. Application Data Primary Examiner-Alan L. Rotman Attorney, Agent, or Firm-Nixon & Vanderhye 63 Continuation of Ser. No. 884,641, Jul 11, 1986, aban doned. (57 ABSTRACT The efficacy of immunotoxins having an antibody that 51) Int. Cl.".................. C07D 207/46; CO7D 207/48; recognizes a tumour associated antigen linked to a cyto CO7D 401/12 toxin through a heterobifunctional agent of the disul 52 U.S. Cl. ..................................... 546/281; 548/542 phide type is improved by providing in the heterobi 58 Field of Search ......................... 548/542; 546/281 functional agent a molecular grouping creating steric 56 References Cited hindrance in relation to the disulphide link. -

Converting Disulfide Bridges in Native Peptides to Stable Methylene Thioacetals
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Converting disulfide bridges in native peptides to stable methylene thioacetals† Cite this: Chem. Sci.,2016,7, 7007 C. M. B. K. Kourra and N. Cramer* Disulfide bridges play a crucial role in defining and rigidifying the three-dimensional structure of peptides. However, disulfides are inherently unstable in reducing environments. Consequently, the development of strategies aiming to circumvent these deficiencies – ideally with little structural disturbance – are highly sought after. Herein, we report a simple protocol converting the disulfide bond of peptides into highly stable methylene thioacetal. The transformation occurs under mild, biocompatible conditions, enabling the conversion of unprotected native peptides into analogues with enhanced stability. The developed Received 23rd May 2016 protocol is applicable to a range of peptides and selective in the presence of a multitude of potentially Accepted 24th July 2016 reactive functional groups. The thioacetal modification annihilates the reductive lability and increases the DOI: 10.1039/c6sc02285e serum, pH and temperature stability of the important peptide hormone oxytocin. Moreover, it is shown www.rsc.org/chemicalscience that the biological activities for oxytocin are retained. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction disulde bond engineering emerged as an important strategy to improve the metabolic stability of disulde-containing peptides, Peptides have recently been enjoying a renewed interest in whilst maintaining their biological activity. For instance, their application as therapeutic agents.1 They provide a large replacements of the disulde group with a lactam,10 thioether,11 a chemical space with a diverse array of molecular frameworks selenium12 or dicarba13 analogues have been reported.5 Many of for the development of novel therapeutics for a plethora of these methods require signicant modication of the synthetic biomedical applications. -

Isolation and Structure Elucidation of Pyridine Alkaloids from the Aerial
www.nature.com/scientificreports OPEN Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge Dumaa Mishig1,2,3, Margit Gruner1, Tilo Lübken1, Chunsriimyatav Ganbaatar1,2, Duger Regdel2 & Hans‑Joachim Knölker1* The seven pyridine alkaloids 1–7, the favonoid acacetin (8), and L‑proline anhydride (9) have been isolated from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. The structures of the natural products 1–9 have been assigned by MS, as well as IR, 1D NMR (1H, 13C, DEPT), and 2D NMR (COSY, HSQC, HMBC, NOESY) spectroscopic methods. The compounds 2 and 4–7 represent new chemical structures. Acacetin (8) and L‑proline anhydride (9) have been obtained from C. mongolica for the frst time. Caryopteris mongolica Bunge is a deciduous shrub and belongs to the Verbenaceae family. C. mongolica which is widely distributed in the mountainous and Gobi regions of Mongolia (Khentei, Khangai, Mongol-Daurian, Mid- dle Khalkha, Mongolian Altai, East Mongolia Valley of Lakes, Govi-Altai, East Govi, Trans-Altai Gobi, Gobi and Alashan Gobi)1. In fact only this species of Caryopteris is growing in Mongolia, whereas about 16 species of this genus occur all over the world. In traditional Mongolian medicine, the aerial parts of this plant have been prepared as decoction and used for haemorrhage, increasing muscle strength, urinary excretion, pulmonary windy oedema and chronic bronchitis2. In Chinese folk medicine, Caryopteris ternifora has been used as anti- pyretic, detoxifying, expectorant, and anti-infammatory agent and for the treatment of cold, tuberculosis and rheumatism3. -

Bioresorbable Stereochemically Defined Polymers for Tissue Engineering and Wireless Bio-Integrated Electronic Device Applications
© 2021 Yen-Hao Hsu ALL RIGHTS RESERVED BIORESORBABLE STEREOCHEMICALLY DEFINED POLYMERS FOR TISSUE ENGINEERING AND WIRELESS BIO-INTEGRATED ELECTRONIC DEVICE APPLICATIONS A Dissertation Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Yen-Hao Hsu March, 2021 BIORESORBABLE STEREOCHEMICALLY DEFINED POLYMERS FOR TISSUE ENGINEERING AND WIRELESS BIO-INTEGRATED ELECTRONIC DEVICE APPLICATIONS Yen-Hao Hsu Dissertation Approved: Accepted: _______________________________ ______________________________ Advisor Interim Director of SPSPE Dr. Matthew L. Becker Dr. Ali Dhinojwala _______________________________ ______________________________ Committee Member Interim Dean of the College Dr. Yu Zhu Dr. Craig Menzemer _______________________________ ______________________________ Committee Member Interim Director, Graduate School Dr. Chrys Wesdemiotis Dr. Marnie Saunders _______________________________ ______________________________ Committee Member Date Dr. Xiong Gong _______________________________ Committee Member Dr. Kevin A. Cavicchi iii ABSTRACT In most synthetic bioresorbable polymers, changing the physical properties such as elasticity and toughness by monomers results in a change to the crystallinity of the material, which manifests through alteration of its mechanical performance. “Thiol-yne” click chemistry has been discovered as an efficient methodology for step-growth polymerization between thiols and activated alkynes. Variation of the solvent polarity -

Bio-Based Reactive Diluents and Thiol-Ene
BIO-BASED REACTIVE DILUENTS AND THIOL-ENE PHOTOPOLYMERIZATION FOR ENVIRONMENTALLY BENIGN COATINGS A Dissertation Presented to The Graduate Faculty of the University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Kosin Wutticharoenwong December, 2007 BIO-BASED REACTIVE DILUENTS AND THIOL-ENE PHOTOPOLYMERIZATION FOR ENVIRONMENTALLY BENIGN COATINGS Kosin Wutticharoenwong Dissertation Approved: Accepted: _____________________________ _____________________________ Advisor Department Chair Dr. Mark D. Soucek Dr. Sadhan C. Jana _____________________________ _____________________________ Committee Member Dean of the College Dr. Kevin Cavicchi Dr. Stephen Cheng _____________________________ _____________________________ Committee Member Dean of the Graduate School Dr. Kyonsuku Min Cakmak Dr. George R. Newkome _____________________________ _____________________________ Committee Member Date Dr. George Chase _____________________________ Committee Member Dr. Wiley J. Youngs ii ABSTRACT Tung oil was used as diene for modification via a Diels-Alder reaction with acrylate dienophiles. Tung oil was modified with three different acrylate molecules: 3- methacryloxypropyl trimethoxysilane (MAS), 2,2,2-trifluoroethyl methacrylate (TFM) and triallyl ether acrylate (TAEA) at atmospheric pressure. The modified tung oils were characterized using 1H NMR, 13C NMR, and FT-IR. The molecular weight and distribution were characterized using GPC, and MALDI-TOF. The effects of new acrylate modified tung oils on the properties of alkyd-based coatings film were investigated including hardness, solvent resistance, flexibility, gloss, impact resistance, contact angle, tensile, and thermo-mechanical properties. The viscosity can be reduced to the application viscosity of the alkyd by the reactive diluents. Drying time study showed that drying time can be altered by types and level of diluent added. All the results revealed that modified tung oils can be used as volatile organic compound (VOC) compliant in alkyd systems. -

1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes >11 million organic compounds which are classified into families according to structure and reactivity Functional Group (FG): group of atoms which are part of a large molecule that have characteristic chemical behavior. FG’s behave similarly in every molecule they are part of. The chemistry of the organic molecule is defined by the function groups it contains 1 C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic C N C C C X X= F, Cl, Br, I amines Alkenes Alkyl Halide H C C C O C C O Alkynes alcohols ethers acidic H H H C S C C C C S C C H sulfides C C thiols (disulfides) H H Arenes Carbonyl-oxygen double bonds (carbonyls) Carbon-nitrogen multiple bonds acidic basic O O O N H C H C O C Cl imine (Schiff base) aldehyde carboxylic acid acid chloride O O O O C C N C C C C O O C C nitrile (cyano group) ketones ester anhydrides O C N amide opsin Lys-NH2 + Lys- opsin H O H N rhodopsin H 2 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon and hydrogen Saturated" contains only single bonds Isomers: compounds with the same chemical formula, but different arrangement of atoms Constitutional isomer: have different connectivities (not limited to alkanes) C H O C4H10 C5H12 2 6 O OH butanol diethyl ether straight-chain or normal hydrocarbons branched hydrocarbons n-butane n-pentane Systematic Nomenclature (IUPAC System) Prefix-Parent-Suffix -

Ncomms9697.Pdf
ARTICLE Received 20 Jul 2015 | Accepted 16 Sep 2015 | Published 29 Oct 2015 DOI: 10.1038/ncomms9697 OPEN Efficient purification of ethene by an ethane-trapping metal-organic framework Pei-Qin Liao1, Wei-Xiong Zhang1, Jie-Peng Zhang1 & Xiao-Ming Chen1 Separating ethene (C2H4) from ethane (C2H6) is of paramount importance and difficulty. Here we show that C2H4 can be efficiently purified by trapping the inert C2H6 in a judiciously designed metal-organic framework. Under ambient conditions, passing a typical cracked gas mixture (15:1 C2H4/C2H6) through 1 litre of this C2H6 selective adsorbent directly produces 56 litres of C2H4 with 99.95% þ purity (required by the C2H4 polymerization reactor) at the outlet, with a single breakthrough operation, while other C2H6 selective materials can only produce ca. p1 litre, and conventional C2H4 selective adsorbents require at least four adsorption–desorption cycles to achieve the same C2H4 purity. Single-crystal X-ray diffraction and computational simulation studies showed that the exceptional C2H6 selectivity arises from the proper positioning of multiple electronegative and electropositive functional groups on the ultramicroporous pore surface, which form multiple C–H ÁÁÁN hydrogen bonds with C2H6 instead of the more polar competitor C2H4. 1 MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, P.R. China. Correspondence and requests for materials should be addressed to J.-P.Z. (email: [email protected]). NATURE COMMUNICATIONS | 6:8697 | DOI: 10.1038/ncomms9697 | www.nature.com/naturecommunications 1 & 2015 Macmillan Publishers Limited. All rights reserved. -

Reactions of Bifunctional Addition-Fragmentation Chain Transfer Agents for Synthesis of Polymer Bearing Unsaturated Moieties at Both Ends
Macromol. Chem. Phys. 2000, 201, 1565–1573 1565 Full Paper: Polymerizations of methyl methacrylate scopy that unsaturated and seven-membered cyclic end (MMA) and styrene (St) in the presence of bifunctional groups are formed by conventional AFCT and intramole- addition fragmentation chain transfer (AFCT) agents con- cular cyclization of the radical from the transfer agents. sisting of two a-(alkylthiomethyl)acryloyloxy groups con- However, formation of the cyclic end groups could be nected by an alkylene group are presented. The moieties suppressed by structural modification of the transfer from the transfer agent were introduced almost quantita- agent. PMMA bearing the unsaturated end group at one or tively at both ends and at the middle of PMMA under both chain ends was employed as a precursor for branched appropriate conditions. It was confirmed by NMR spectro- block copolymer preparation. Reactions of bifunctional addition-fragmentation chain transfer agents for synthesis of polymer bearing unsaturated moieties at both ends Kenta Tanaka, Bunichiro Yamada* Material Chemistry Laboratory, Faculty of Engineering, Osaka City University, Osaka 558-8585, Japan Fax: +81-6-6605-2797; E-mail: [email protected] Introduction less reactive adduct radical which would readily couple a with PSt radical leading to a star-shaped polymer consist- Some -(substituted methyl)acrylic esters have been [10] known as effective chain transfer agents through an addi- ing of two PMMA chains and up to four PSt chains. tion fragmentation chain transfer (AFCT) mechanism to yield b-carboalkoxyallyl end groups depending on the type of the substituent.[1–4] The same unsaturated end group can be obtained by catalytic chain transfer poly- merization of methyl methacrylate (MMA) using a Co(II) complex.[5] The addition of a PMMA radical to the carbo- methoxyallyl group is not a fast reaction and the adduct radical is known to readily fragment to regenerate the end group and PMMA radical. -

Synthesis and Characterization of Bifunctional Silica Gel Surfaces
Eastern Illinois University The Keep Masters Theses Student Theses & Publications 1996 Synthesis and Characterization of Bifunctional Silica Gel Surfaces Alufelwi Maxwell Tshavhungwe Eastern Illinois University This research is a product of the graduate program in Chemistry at Eastern Illinois University. Find out more about the program. Recommended Citation Tshavhungwe, Alufelwi Maxwell, "Synthesis and Characterization of Bifunctional Silica Gel Surfaces" (1996). Masters Theses. 1857. https://thekeep.eiu.edu/theses/1857 This is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. THESIS REPRODUCTION CERTIFICATE TO: Gr~duate Degree Candidates (who have written formal theses) SUBJECT: Permission to Reproduce Theses The University Library is rece1v1ng a number of requests from other institutions asking permission to reproduce dissertations for inclusion in their library holdings. Although no copyright laws are involved, we feel that professional courtesy demands that permission be obtained from the author before we allow theses to be copied. PLEASE SIGN ONE OF THE FOLLOWING STATEMENTS: Booth Library of Eastern Illinois University has my permission to lend my thesis to a reputable college or university for the purpose of copying it for inclusion in that institution's library or research holdings. Date I respectfully request Booth Library of Eastern Illinois University not allow my thesis to be reproduced because: ut or Date SYNTHESIS AND CHARACTERIZATION OF BIFUNCTIONAL SILICA GEL SURFACES (TITLE) BY ALUFELWI MAXWELL TSHAVHUNGWE THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTERS OF SCIENCE IN CHEMISTRY IN THE GRADUATE SCHOOL, EASTERN ILLINOIS UNIVERSITY CHARLESTON. -

Aldehydes, Ketones and Carboxylic Acids
1212Unit Objectives AldehydesAldehydesAldehydesAldehydes,,,,,, KKKKKKeeeeeetonestonestonestonestonestones After studying this Unit, you will be able to andandandandandand CarboxylicCarboxylicCarboxylicCarboxylicCarboxylicCarboxylic • write the common and IUPAC names of aldehydes, ketones and carboxylic acids; AAAAAAcidscidscidscidscidscids • write the structures of the compounds containing functional groups namely carbonyl and carboxyl groups; Carbonyl compounds are of utmost importance to organic chemistry. They are constituents of fabrics, flavourings, plastics • describe the important methods and drugs. of preparation and reactions of these classes of compounds; In the previous Unit, you have studied organic • correlate physical properties and compounds with functional groups containing carbon- chemical reactions of aldehydes, oxygen single bond. In this Unit, we will study about the ketones and carboxylic acids, organic compounds containing carbon-oxygen double with their structures; bond (>C=O) called carbonyl group, which is one of the • explain the mechanism of a few most important functional groups in organic chemistry. selected reactions of aldehydes and ketones; In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in the ketones, it is bonded • understand various factors to two carbon atoms. The carbonyl compounds in which affecting the acidity of carboxylic carbon of carbonyl group is bonded to carbon or acids and their reactions; hydrogen and oxygen of hydroxyl moiety (-OH) are • describe the uses of aldehydes, known as carboxylic acids, while in compounds where ketones and carboxylic acids. carbon is attached to carbon or hydrogen and nitrogen of -NH2 moiety or to halogens are called amides and acyl halides respectively. Esters and anhydrides are derivatives of carboxylic acids. The general formulas of these classes of compounds are given below: 2021–22 Aldehydes, ketones and carboxylic acids are widespread in plants and animal kingdom. -

Trichilianones A-D, Novel Cyclopropane-Type Limonoids from Trichilia Adolfi
molecules Article Trichilianones A-D, Novel Cyclopropane-Type Limonoids from Trichilia adolfi Ivan Limachi 1,2 , Mariela Gonzalez-Ramirez 1, Sophie Manner 1 , Juan C. Ticona 2, Efrain Salamanca 2, Alberto Gimenez 2 and Olov Sterner 1,* 1 Department of Chemistry, Centre for Analysis and Synthesis, Lund University, 22100 Lund, Sweden; [email protected] (I.L.); [email protected] (M.G.-R.); [email protected] (S.M.) 2 Instituto de Investigaciones Farmaco Bioquimicas, Universidad Mayor de San Andres, La Paz, Bolivia; [email protected] (J.C.T.); [email protected] (E.S.); [email protected] (A.G.) * Correspondence: [email protected]; Tel.: +46-70-5306649 Abstract: The fractionation of an ethanol extract of the bark of Trichilia adolfi yielded four novel limonoids (trichilinones A-D, 1–4), with five fused rings and related to the hortiolide-type limonoids. Starting with an "-lactone, which is α,β-unsaturated in trichilinones A and D (1 and 4), attached to a tetrahydrofuran ring that is connected to an unusual bicyclo [5.1.0] hexane system, joined with a cyclopentanone with a 3-furanyl substituent [(2-oxo)-furan-(5H)-3-yl in trichilinone D (4)], the four compounds isolated display a new 7/5/3/5/5 limonoid ring system. Their structures were established based on extensive analysis of NMR spectroscopic data. As the crude extract possessed anti-leishmanial properties, the compounds were assayed for cytotoxic and anti-parasitic activities in vitro in murine macrophages cells (Raw 264.7) and leishmania promastigotes (L. amazoniensis Citation: Limachi, I.; and L.