Research and Teaching Report Bst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arqueologia De La Batalla De Catalunya (1939)
EBRE 38 193 2- 2010 ARQUEOLOGIA DE LA BATALLA DE CATALUNYA (1939). EXCAVACIONS D’UN TRAM DE LA LÍNEA DEFENSIVA L-3 A SUBIRATS (ALT PENEDÈS, BARCELONA). ARCHAEOLOGY OF THE BATTLE OF CATALONIA (1939). EXCAVATIONS OF A SECTION OF THE DEFENSIVE LINE L-3 IN SUBIRATS (ALT PENEDÈS, BARCELONA). M. Carmen Rojo-Ariza1*, Ramon Arnabat Mata**, Gemma Cardona-Gómez*, David Íñiguez-Gràcia*, Ignasi Fernández-Aubareda*** *Grup de recerca DIDPATRI (SGR 2009-00245) Departament de Didàctica de les Cièncias Socials Facultat de Formació del Professorat Passeig de la Vall d’Hebron, 171 Despatx 115, Planta 1, Edifici de Llevant Universitat de Barcelona. 08035. Barcelona [email protected], [email protected], [email protected]. ** Departament d’Història i Història de l’Art Facultat de Lletres Universitat Rovira i Virgili Avinguda Catalunya, 35. 43002 [email protected]. ***Arqueòleg c/ Carles Riba, 3. 08720. Vilafranca del Penedès. Barcelona. [email protected] Rebut: 30/10/2010 Acceptat: 08/11/2010 Resum En aquest article descrivim els resultats de la intervenció arqueològica realitzada en les restes de les estructures defensives de la Guerra Civil espanyola al poble de Sant Pau d’Ordal, Subirats (Alt Penedès, Barcelona) que van estar operatives en les darreres fases del conflicte a Catalunya (gener de 1939). Tot i l’elevat nombre d’aquestes, cap dels elements excavats proporcionà un nombre significatiu de materials de l’època. Per això, reflexionem també aquí sobre la problemàtica de l’escàs grau de protecció del patrimoni arqueològic de la Guerra Civil. Paraules clau: Arqueologia del conflicte, Batalla de Catalunya, trinxeres, niu de metralladora, espoli. -

Regional Aid Map 2007-2013 EN
EUROPEAN COMMISSION Competition DG Brussels, C(2006) Subject: State aid N 626/2006 – Spain Regional aid map 2007-2013 Sir, 1. PROCEDURE 1. On 21 December 2005, the Commission adopted the Guidelines on National Regional Aid for 2007-20131 (hereinafter “RAG”). 2. In accordance with paragraph 100 of the RAG, each Member State should notify to the Commission, following the procedure of Article 88(3) of the EC Treaty, a single regional aid map covering its entire national territory which will apply for the period 2007-2013. In accordance with paragraph 101 of the RAG, the approved regional aid map is to be published in the Official Journal of the European Union and will be considered as an integral part of the RAG. 3. On 13 March 2006, a pre-notification meeting between the Spanish authorities and the Commission's services took place. 4. By letter of 19 September 2006, registered at the Commission on the same day with the reference number A/37353, Spain notified its regional aid map for the period from 1 January 2007 to 31 December 2013. 5. By letter of 23 October 2006 (reference number D/59110) the Commission requested from the Spanish authorities additional information. 6. By letter of 15 November 2006, registered at the Commission with the reference number A/39174, the Spanish authorities submitted additional information. 1 OJ C 54, 4.3.2006, p. 13. 2. DESCRIPTION 2.1. Main characteristics of the Spanish Regional aid map 7. Articles 40(1) and 138(1) of the Spanish Constitution establish the obligation of the public authorities to look after a fair distribution of the wealth among and a balanced development of the various parts of the Spanish territory. -
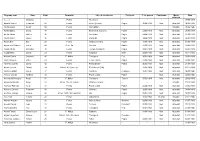
Cognom, Nom Nom Edat Cementiri Lloc De Residència Professió C. De
Cognom, nom Nom Edat Cementiri Lloc de residència Professió C. de guerra Condemna Motiu Data defunció (..., ...), Domingo - Fossa No consta - - - - 03/05/1939 Abadia Gómez Francesc 64 Fossa Falset (Priorat) Pagès 10/04/1939 Mort Afusellat 06/05/1939 Abellà Costa Serafí 55 Fossa Móra d'Ebre - - - Malaltia 05/02/1940 Abella Mateu Josep 46 Fossa Barberà de la Conca Pagès 28/02/1939 Mort Afusellat 20/03/1939 Abellà Trillas Maties 36 Fossa Montblanc Pagès 24/04/1939 Mort Afusellat 31/05/1939 Abelló Cabré Jaume 36 Fossa Poboleda Pagès 14/04/1939 Mort Afusellat 06/05/1939 Adrian Bes Blai 28 Fossa Vilalba dels Arcs Pagès 11/05/1939 Mort Afusellat 15/06/1939 Agustench Solanes Josep 49 Secc. 5a Mont-ral Barber 12/05/1939 Mort Afusellat 08/08/1939 Aixalà Abelló Salvador 30 Fossa Torroja del Priorat Pagès 09/05/1939 Mort Afusellat 14/07/1939 Aixalà Masó Josep 29 Fossa Amposta Xofer 20/07/1939 Mort Afusellat 16/11/1939 Alabau Garcia Sebastià 45 P. Militar Tarragona Peó 16/02/1939 Mort Afusellat 28/02/1939 Alba Carbonell Rafel 23 Fossa Jesús i Maria Pagès 21/02/1940 Mort Afusellat 20/06/1941 Alberich Cubells Josep 42 Fossa Palma d'Ebre Paleta 06/06/1939 Mort Afusellat 21/07/1939 Albert Colomé Dionisi 29 Nínxol 40 - Secc. 6a El Catllar de Gaià - 11/08/1939 Mort Afusellat 15/11/1939 Albiñana Matas Josep 37 Fossa Cambrils Perruquer 16/01/1940 Mort Afusellat 04/09/1940 Alcover Llorens Ambrosi 32 Fossa Pla de Cabra Pagès - Mort Afusellat 24/08/1940 Alcoverro Berenguer Àngel 54 P. -

Segregacions: Un Problema De Fronteres
SEGREGACIONS: UN PROBLEMA DE FRONTERES ESTUDI HISTÒRICO-JURÍDIC A TRAVÉS DEL PERELLÓ I LES TERRES DE L’EBRE El Perelló 2014 1 2 ÍNDEX Relació d'abreviatures pàg. 5 1. Introducció pàg. 7 2. Origen i evolució del municipi pàg.13 2.1 Teories pàg.13 2.2 Evolució dels municipis a les Terres de l'Ebre pàg.14 3. El municipi liberal pàg.19 4. Segregacions durant el segle XIX pàg.21 4.1 Marc legal pàg.21 4.2 Enumeració i descripció essencial de les segregacions a les pàg.27 Terres de l'Ebre 4.3 Anàlisi concret de la segregació de l'Ametlla de Mar pàg.28 respecte del Perelló 5. Segregacions durant el segle XX pàg.37 5.1 Marc legal pàg.37 5.2 Enumeració i descripció essencial de les segregacions a les pàg.42 Terres de l'Ebre 5.3Anàlisi concret de la segregació de l'Ampolla respecte del Perelló pàg.45 6. Comparativa entre segregacions del s. XIX respecte al s. XX pàg.61 Legislació aplicable Parts legitimades per instar la segregació Òrgan competent Requisits legals Participació dels actors en el procés Adequació a la realitat social 7. Consideracions finals pàg.67 8. Bibliografia pàg.69 9. Fonts documentals pàg.73 Annexos pàg.75 3 4 Relació d'abreviatures BOE Butlletí Oficial de l'Estat CE Constitució Espanyola DOGC Diari Oficial de la Generalitat de Catalunya FJ Fonament Jurídic Sr Senyor TC Tribuna Constitucional TS Tribunal Suprem Pàg. Pàgina Pàgs. Pàgines 5 6 1. INTRODUCCIÓ En l'actualitat ens trobem immersos en una greu crisi econòmica que ha provocat que es qüestioni l'organització i el model de la societat contemporània. -

Medieval Church Located on the Highest Part of Querol. It Has
La primera referència documental de Querol la trobem en una carta del rei Lotari, datada l’any 988. El 993 era propietat del vicari comtal de Gurb que, en el seu tes- tament, deixà algunes terres dels seus castells de Montagut, Querol i Pinyana al Monestir de Sant Cugat. L’any 996 fou venut a la casa de Cervelló i el 1053, Guerau Alemany de Cervelló posà en homenatge i sota l’autoritat de Ramon Berenguer I de Barcelona, els tres castells esmentats, a més del de Pontils. Aquest homenatge fou repetit el 1062. Els castells formaven part de la baronia de Cervelló o de la Lla- cuna, i cap a la fi del segle XIII el castell de Querol esdevingué el centre de la Baronia d’aquest nom. L’esmentada Baronia es- tigué de sempre vinculada als Cervelló, dels quals passà vers el 1528 a la família Barberà, castlans de Vilafranca del Penedès, i cap al 1597 als Saiol, els quals la posseïren fins al segle XIX. El Castell de Querol sofrí amb el temps diverses vicissituds Església de Santa Maria - Situada a la part alta del nucli de i, finalment, el 1835, una partida de liberals el prengueren i el Querol, tot i haver sofert diverses modificacions, és d’origen van enderrocar quan estava ocupat per un grup carlí. medieval i està formada per una sola nau amb volta apuntada. Església de Sant Jaume de Montagut - Situada al peu La primera referencia documental de Querol la encontramos en del cim de Montagut, es tracta d’una construcció gòtica, tot una carta del rey Lotari, fechada el año 988. -

Especial Especial Els Textos Enviats D’Opinió Iparticipaciópoden Sofrirmodificacions O Retalls Permotius D’Espai
Març 2015 - Especial especial SUMARI Editorial ............................. 3 L’Ajuntament de Santa Bàrbara sanejat ANUNCIANTS econòmicament ...................... 22 Remodelació del Parc Municipal ......... 4 LA PLANA, SL Camins del terme municipal El nou Mercat Municipal enllestit ........ 6 RAMON TAFALLA de Santa Bàrbara ..................... 23 Smartcentre Santa Bàrbara ............. 8 CONTREGISA Construcció d’un nou magatzem ....... 23 El nou Centre Municipal IMPREMTA QUEROL Nou Servei de Teràpia Ocupacional SOREA de Serveis Socials .................... 10 d’APASA ............................ 23 LA CAIXA Rehabilitació del Cementiri Municipal ... 12 Activitats al Centre Obert l’Oliveta ...... 24 MARTORELL RUBIO ASSESSORS Arranjament del tram final El petit hotel d’entitats a la ELECTRICITAT ROYO ANDREU del passeig de les Escoles .............. 13 casa del carrer Ametller ............... 24 NEMEA Canalització del barranquet ........... 14 Festivitat de Sant Gregori .............. 25 PRONTOSERVIS 18 anys de la Fira de l’Oli Novell, Activitats Serveis Socials i Salut ........ 25 ÒSCAR MARCO dels Cítrics i del Comerç ............... 16 Millores a les instal·lacions CAMPO Ampliació de l’Escola Jaume Balmes .... 17 de les piscines municipals ............. 26 HOTEL DIEGO Inauguració dels nous Mèrits Planers ....................... 26 MONTEBRE SCCL estudis de la Plana Ràdio .............. 18 Entrega de la beca IES les Planes ........ 27 AGROIGE URBANITZACIÓ LA PLANA Parc de la Independència .............. 19 Treball i Formació .................... 27 AGÈNCIA DE RESIDUS DE Remodelació de la plaça del dipòsit ..... 20 Alcaldia i Urbanisme ................. 28 CATALUNYA Activitats del Grup Joventut ............ 20 Agricultura ......................... 30 CARNS ALBESA El fons de l’Ajuntament de Benestar Social, Joventut i Salut ........ 31 TRANSFORMACIONS AGRÀRIES Santa Bàrbara ingressa a Cultura i Ensenyament ............... 35 MARTÍ SL l’Arxiu Comarcal del Montsià .......... 21 Esports i Medi Ambient .............. -

Actes Dont La Publication Est Une Condition De Leur Applicabilité)
30 . 9 . 88 Journal officiel des Communautés européennes N0 L 270/ 1 I (Actes dont la publication est une condition de leur applicabilité) RÈGLEMENT (CEE) N° 2984/88 DE LA COMMISSION du 21 septembre 1988 fixant les rendements en olives et en huile pour la campagne 1987/1988 en Italie, en Espagne et au Portugal LA COMMISSION DES COMMUNAUTÉS EUROPÉENNES, considérant que, compte tenu des donnees reçues, il y a lieu de fixer les rendements en Italie, en Espagne et au vu le traité instituant la Communauté économique euro Portugal comme indiqué en annexe I ; péenne, considérant que les mesures prévues au présent règlement sont conformes à l'avis du comité de gestion des matières vu le règlement n0 136/66/CEE du Conseil, du 22 grasses, septembre 1966, portant établissement d'une organisation commune des marchés dans le secteur des matières grasses ('), modifié en dernier lieu par le règlement (CEE) A ARRÊTÉ LE PRESENT REGLEMENT : n0 2210/88 (2), vu le règlement (CEE) n0 2261 /84 du Conseil , du 17 Article premier juillet 1984, arrêtant les règles générales relatives à l'octroi de l'aide à la production d'huile d'olive , et aux organisa 1 . En Italie, en Espagne et au Portugal, pour la tions de producteurs (3), modifié en dernier lieu par le campagne 1987/ 1988 , les rendements en olives et en règlement (CEE) n° 892/88 (4), et notamment son article huile ainsi que les zones de production y afférentes sont 19 , fixés à l'annexe I. 2 . La délimitation des zones de production fait l'objet considérant que, aux fins de l'octroi de l'aide à la produc de l'annexe II . -

Els Cervelló, Barons De Querol-Montagut a Cedat Mitjana
ELS CERVELLÓ, BARONS DE QUEROL-MONTAGUT A CEDAT MITJANA Una de les grans famíllies nobles establertes al Penedes des dels inicis del repoblament de la comarca foren els Cervelló, un llinatge aristocratic que senyoreja aquestes terres durant tota I'edad mitjana. En el temps es va dividir en dues grans estirps, una de les quals fou la dels barons de Querol i Montagut. Aquesta comunicació és la continuació de la presentada I'any paSSat i que portava el titol ((Estrategies senyorials per a la forrnació d'un dornini: els Cervelló a I'edat rnitjana>>.(') ELS CERVELLÓ, BARONS DE QUEROL-MONTAGUT A L'EDAT MITJANA L'estudi del llinatge dels Cervelló permet descobrir una família destacada dins I'aristocracia catalana medieval i de cabdal importan- cia dins les terres de la nostra comarca. Com varem veure I'any passat, els Cervelló són un llinatge iniciat el segle x amb Ansulf i (mort I'any 942) i el seu fill Ansulf ii (mort entre el 972/986), els quals, a més d'ésser vicaris de Gurb a Osona, aprisio- naren terres a la conca alta del riu Gaia, seguint I'expansió cap al sud, i esdevingueren senyors de Montagut, Querol, Pinyana, Selmella i Santa Perpetua. Aquests personatges són els caps del llinatge dels Gurb-Queralt, una de les famílies més importants i protagonista desta- cada a la repoblació del comtat d10sona-Manresa. El fill segon d'Ansulf ii fou Hug i de Montagut i de Cervelló, que es casa amb Eliarda de Gelida, filla i hereva d'Ennec Bonfill, que pertanyia al llinatge vescomtal. -

Phenomenology of High-Ozone Episodes in NE Spain
Atmos. Chem. Phys., 17, 2817–2838, 2017 www.atmos-chem-phys.net/17/2817/2017/ doi:10.5194/acp-17-2817-2017 © Author(s) 2017. CC Attribution 3.0 License. Phenomenology of high-ozone episodes in NE Spain Xavier Querol1, Gotzon Gangoiti2, Enrique Mantilla3, Andrés Alastuey1, Maria Cruz Minguillón1, Fulvio Amato1, Cristina Reche1, Mar Viana1, Teresa Moreno1, Angeliki Karanasiou1, Ioar Rivas1, Noemí Pérez1, Anna Ripoll1, Mariola Brines1, Marina Ealo1, Marco Pandolfi1, Hong-Ku Lee4, Hee-Ram Eun4, Yong-Hee Park4, Miguel Escudero5, David Beddows6, Roy M. Harrison6,a, Amelie Bertrand7, Nicolas Marchand7, Andrei Lyasota8,†, Bernat Codina8, Miriam Olid8, Mireia Udina8, Bernat Jiménez-Esteve8, María R. Soler8, Lucio Alonso2, Millán Millán3, and Kang-Ho Ahn4 1Institute of Environmental Assessment and Water Research, IDAEA-CSIC, C/Jordi Girona 18–26, 08034 Barcelona, Spain 2Escuela Técnica Superior Ingeniería de Bilbao, Departamento Ingeniería Química y del Medio Ambiente, Universidad del País Vasco UPV/EHU, Urkixo Zumarkalea, S/N, 48013 Bilbao, Spain 3Centro de Estudios Ambientales del Mediterráneo, CEAM, Unidad Asociada al CSIC, Parque Tecnológico C/Charles R. Darwin, 14 46980 Paterna, Valencia, Spain 4Department of Mechanical Engineering, Hanyang University, Ansan 425-791, Republic of Korea 5Centro Universitario de la Defensa de Zaragoza, Academia General Militar, Ctra. de Huesca s/n, 50090 Zaragoza, Spain 6Division of Environmental Health & Risk Management, School of Geography, Earth & Environmental Sciences, University of Birmingham, Edgbaston, -

Pdf Nuevas Centralidades En El Territorio Del Delta Del Ebro / Vicent
NUEVAS CENTRALIDADES EN EL TERRITORIO DEL DELTA DEL EBRO VICENT ORTELLS CABRERA Y ANTONIO QUEROL GÓMEZ Universitat Jaume I y Universitat Internacional de Catalunya La nueva redistribución territorial de Catalunya, actualmente en estudio y elabora- ción por parte de la Generalitat, dividirá administrativamente en seis nuevos territo- rios denominados veguerías. Ampliará pues, las actuales cuatro provincias y reapare- ce un territorio histórico, la antigua veguería del Ebro, que aglutina las tierras catala- nas bañadas por este río. Estas comarcas, la Terra Alta, Ribera d’Ebre, Baix Ebre y Montsià, forman un conjunto diferenciado del resto de Catalunya tanto por su historia (prácticamente todas o gran parte de ellas, formaron parte del territorio jurídico y administrativo de la ciudad de Tortosa, a raíz de la conquista cristiana por parte del monarca catalano- aragonés Ramón Berenguer IV), como por la lengua (la variante occidental del cata- lán), economía (generalmente primaria y localizada por el curso fluvial que favoreció los intercambios) y la percepción ( el propio curso fluvial), además de las costumbres e idiosincrasia. Territorio con status político propio durante siglos, también conocido como “cruïlla dels països catalans”, y salida al mar de las tierras de poniente e inte- riores de la antigua Corona de Aragón. Este conjunto comarcal del Ebro, se prolonga también por historia, sociedad, eco- nomía y percepción geográfica, hacia otras zonas próximas de Aragón y València, como ocurre con las tierras del Matarranya en Teruel y el Maestrat de Castelló. Gran importancia para ello tuvo desde siempre la jerarquía eclesiástica de Tortosa, ya que hasta hace pocos años su diócesis se extendía hasta la propia Plana de Castelló y man- tiene hoy en día las comarcas del Maestrat y Ports en esta provincia. -

L'alt Camp a L'alt Camp, El Pla Territorial Aposta Perquè La Capital
L’Alt Camp A l’Alt Camp, el Pla territorial aposta perquè la capital comarcal esdevingui una ciutat de molt major pes que l’actual, capaç de portar serveis de primer ordre a la seva comarca, d’interconnectar-se amb la conurbació central Tarragona - Reus i de situar-se en el mapa del transport ferroviari. Si Valls no fa un salt important, quedarà inevitablement despenjada de la xarxa urbana i infraestructural principal de la regió. Ara mateix, determinades apostes ferroviàries a la comarca són inviables i només en un altre escenari de poblament podrien ser formulades més enllà de l’escenari temporal del Pla. En conseqüència, s’assenyala per a Valls una estratègia de creixement potenciat i s’insta el planejament urbanístic a fer una aposta contundent i decidida en benefici del conjunt de la comarca. L’estratègia d’espais oberts i de noves infraestructures està en consonància amb aquesta voluntat del Pla. Tanmateix, i a l’entorn de Valls, convindria intensificar la disciplina urbanística en el sòl no urbanitzable tenint en compte que es tracta d’una plana agrícola de gran valor paisatgístic que té una densitat d’edificacions de tota mena superior a la que seria desitjable. El Pla proposa l’estratègia de creixement mitjà, atès el paper nodal que juguen, l’adequació dels terrenys confrontants, la posició territorial i l’accessibilitat per als nuclis d’Alcover, el Pla de Santa Maria, Rodonyà i Vila-rodona. El Pla proposa l’estratègia de creixement moderat, amb l’objectiu que es puguin atendre les necessitats endògenes i fins atreure una petita quantitat de població forània, per als nuclis d’Alió, Bràfim, Cabra del Camp, el Milà, el Pla de Manlleu, el Pont d'Armentera, el Rourell, Figuerola del Camp, la Masó, la Riba, les Pobles, Mont-ral, Montferri, Nulles, Picamoixons, Puigpelat, Querol, Vallmoll i Vilabella. -

24, 48 and 72 Hours at Costa Daurada
ENG @costadauradatur 24, 48 and 72 hours at Costa Daurada Table1 Discover Salou ••of Content 4-9 2 Dive into the Costa Daurada •• 10 3 Live PortAventura World ••• 12 4 Hail Caesar • 14 5 Follow the footsteps of the Iberians •• 16 6 Mediterranean architectures • 18 7 Sea lions and pirates •• 20 8 Miró’s corner • 22 9 Roads that talk about wine •• 24 10 Disconnect in Costa Daurada • 26 11 From the orchard to the market • 28 12 The charm of a haunted villa •• 30 13 Ora et labora •• 32 14 Viticulture heritage of the Priorat region •• 34 15 The footprint of prehistory ••• 36 16 The natural modernism of Reus •• 38 17 The Cistercian Route •• 40 18 Map 42 • 24 hours • 48 hours • 72 hours Editted by: Patronat de Turisme de la Diputació de Tarragona. Passeig Torroja, s/n. 43007 Tarragona Tel. (0034) 977 23 03 12 Texts and Project coordination: Vilaniu Comunicació Photographs: Patronat de Turisme de Salou, PortAventura World, Joan Capdevila/PTCD, Kàrting Vendrell, Museu de la Ràdio, Masia Castelló, Patronat de Turisme de Mont-roig Miami, Patronat de Turisme de Vila-seca La Pineda Platja, FEHT, Lydia Plana Valls, Ajuntament de Valls/Pere Toda, Adernats Printing: Anfigraf, S.A. The interior of the cloister of the monastery of Santes Creus Legal deposit: DL T 829-2019 © Jose Carlos León 1 hours24 hours48 We propose a walk around Salou to discover all its must-see places and enjoy a fabulous combination of beaches, architecture, history and memorable scenery. The Jaume I promenade is a very special place, lined with palm trees and Modernist mansions dating from the early twentieth century.