Emerging Evidence on Anemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®)
UnitedHealthcare® Commercial Medical Benefit Drug Policy Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®) Policy Number: 2021D0088F Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Community Plan Policy Coverage Rationale ....................................................................... 1 • Intravenous Iron Replacement Therapy (Feraheme®, Definitions ...................................................................................... 3 Injectafer®, & Monoferric®) Applicable Codes .......................................................................... 3 Background.................................................................................... 4 Benefit Considerations .................................................................. 4 Clinical Evidence ........................................................................... 5 U.S. Food and Drug Administration ............................................. 7 Centers for Medicare and Medicaid Services ............................. 8 References ..................................................................................... 8 Policy History/Revision Information ............................................. 9 Instructions for Use ....................................................................... 9 Coverage Rationale See Benefit Considerations This policy refers to the following intravenous iron replacements: Feraheme® (ferumoxytol) Injectafer® (ferric carboxymaltose) Monoferric® (ferric derisomaltose)* The following -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Mir-205: a Potential Biomedicine for Cancer Therapy
cells Review miR-205: A Potential Biomedicine for Cancer Therapy Neeraj Chauhan 1,2 , Anupam Dhasmana 1,2, Meena Jaggi 1,2, Subhash C. Chauhan 1,2 and Murali M. Yallapu 1,2,* 1 Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, McAllen, TX 78504, USA; [email protected] (N.C.); [email protected] (A.D.); [email protected] (M.J.); [email protected] (S.C.C.) 2 South Texas Center of Excellence in Cancer Research, School of Medicine, University of Texas Rio Grande Valley, McAllen, TX 78504, USA * Correspondence: [email protected]; Tel.: +1-(956)-296-1734 Received: 3 June 2020; Accepted: 21 August 2020; Published: 25 August 2020 Abstract: microRNAs (miRNAs) are a class of small non-coding RNAs that regulate the expression of their target mRNAs post transcriptionally. miRNAs are known to regulate not just a gene but the whole gene network (signaling pathways). Accumulating evidence(s) suggests that miRNAs can work either as oncogenes or tumor suppressors, but some miRNAs have a dual nature since they can act as both. miRNA 205 (miR-205) is one such highly conserved miRNA that can act as both, oncomiRNA and tumor suppressor. However, most reports confirm its emerging role as a tumor suppressor in many cancers. This review focuses on the downregulated expression of miR-205 and discusses its dysregulation in breast, prostate, skin, liver, gliomas, pancreatic, colorectal and renal cancers. This review also confers its role in tumor initiation, progression, cell proliferation, epithelial to mesenchymal transition, and tumor metastasis. -

Allied Health Professionals Consumer Fact Sheet Board of Registration of Allied Health Professionals
Allied Health Professionals Consumer Fact Sheet Board of Registration of Allied Health Professionals The Board of Registration in Allied Health evaluates the qualifications of applicants for licensure and grants licenses to those who qualify. It establishes rules and regulations to ensure the integrity and competence of licensees. The Board is the link between the consumer and the allied health professional and, as such, promotes the public health, welfare and safety. Allied health professionals are occupational therapists and assistants, athletic trainers, and physical therapists and assistants. Occupational Therapists/Occupational Therapist Assistants Occupational therapists are health professionals who use occupational activities with specific goals in helping people of all ages to prevent, lessen or overcome physical, psychological or developmental disabilities. Occupational Therapists and Occupational Therapist Assistants help people with physical, psychological, or developmental problems regain abilities or adjust to handicaps. They work with physicians, physical and speech therapists, nurses, social workers, psychologists, teachers and other specialists. Patients may face handicaps, injuries, illness, psychological or social problems, or barriers due to age, economic, and cultural factors. Occupational Therapists: Consult with treatment teams to develop individualized treatment programs. Select and teach activities based on the needs and capabilities of each patient Evaluate each patient's progress, attitude and behavior. Design special equipment to aid patients with disabilities. Teach patients how to adjust to home, work, and social environments. Test and evaluate patients' physical and mental abilities. Educate others about occupational therapy. The goal of occupational therapy (OT) is for persons to achieve the highest level of independence after an injury or illness. OT addresses the whole person - cognitive, physical and emotional status. -

Massage Therapy Admission Packet
Caldwell Community College and Technical Institute Welcome to Caldwell Community College and Technical Institute. In this packet, you will find information about our Massage Therapy Program including costs, requirements, and details on how to prepare for your new career. Caldwell Campus 2855 Hickory Blvd Hudson, NC 28638 828.726.2200 Watauga Campus 372 Community College Drive Boone, NC 28607 828.297.8120 Amy Swink Massage Therapy Program Coordinator Watauga Campus E-mail: [email protected] Phone: (828)726-2242 and (828) 297-3811 MASSAGE THERAPY COURSE HEA 3021 This course is designed to prepare the student for the certification examination required for the North Carolina licensure application process. Through class work and practical “hands-on” training, students obtain a solid foundation for professional practice as a Massage Therapist. Upon successful completion of the course, the student is eligible to sit for the state exam and apply for state licensure in North Carolina. Competitive Admissions: Students, who successfully complete Massage Therapy training at CCC&TI, will receive two (2) points toward admission into the Physical Therapy Assistant program at CCC&TI Educational Pathway Example: Massage Therapy training > Licensure by examination > Licensed Massage and Bodywork Therapist Estimated Hourly Earnings: According to the Bureau of Labor Statistics website ( www.bls.gov/oes/current/oes319011.htm), current mean annual wage is $44,950. Employment Opportunities: • Chiropractic offices • Home care agencies • Hospice • Hospitals • Medical offices • Retirement homes • Self-employment • Spas FAQs • Is massage therapy training required to become licensed in North Carolina? o Yes. The North Carolina Board of Massage and Bodywork Therapy (NCBMBT) requires an applicant to successfully complete a state approved training program consisting of a minimum 500 in-class training hours. -

Staphylococcus Aureus Bloodstream Infection Treatment Guideline
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI). The recommendations below are guidelines for care and are not meant to replace clinical judgment. The initial page includes a brief version of the guidance followed by a more detailed discussion of the recommendations with supporting evidence. Also included is an algorithm describing management of patients with blood cultures positive for gram-positive cocci. Brief Key Points: 1. Don’t ignore it – Staphylococcus aureus isolated from a blood culture is never a contaminant. All patients with S. aureus in their blood should be treated with appropriate antibiotics and evaluated for a source of infection. 2. Control the source a. Removing infected catheters and prosthetic devices – Retention of infected central venous catheters and prosthetic devices in the setting of S. aureus bacteremia (SAB) has been associated with prolonged bacteremia, treatment failure and death. These should be removed if medically possible. i. Retention of prosthetic material is associated with an increased likelihood of SAB relapse and removal should be considered even if not clearly infected b. Evaluate for metastatic infections (endocarditis, osteomyelitis, abscesses, etc.) – Metastatic infections and endocarditis are quite common in SAB (11-31% patients with SAB have endocarditis). i. All patients should have a thorough history taken and exam performed with any new complaint evaluated for possible metastatic infection. ii. Echocardiograms should be strongly considered for all patients with SAB iii. All patients with a prosthetic valve, pacemaker/ICD present, or persistent bacteremia (follow up blood cultures positive) should undergo a transesophageal echocardiogram 3. -
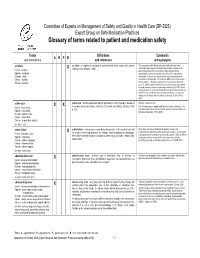
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -

Iron Deficiency Anemia: Evaluation and Management MATTHEW W
Iron Deficiency Anemia: Evaluation and Management MATTHEW W. SHORT, LTC, MC, USA, and JASON E. DOMAGALSKI, MAJ, MC, USA Madigan Healthcare System, Tacoma, Washington Iron deficiency is the most common nutritional disorder worldwide and accounts for approxi- mately one-half of anemia cases. The diagnosis of iron deficiency anemia is confirmed by the findings of low iron stores and a hemoglobin level two standard deviations below normal. Women should be screened during pregnancy, and children screened at one year of age. Supple- mental iron may be given initially, followed by further workup if the patient is not responsive to therapy. Men and postmenopausal women should not be screened, but should be evaluated with gastrointestinal endoscopy if diagnosed with iron deficiency anemia. The underlying cause should be treated, and oral iron therapy can be initiated to replenish iron stores. Paren- teral therapy may be used in patients who cannot tolerate or absorb oral preparations. (Am Fam Physician. 2013;87(2):98-104. Copyright © 2013 American Academy of Family Physicians.) ▲ Patient information: ron deficiency anemia is diminished red causes of microcytosis include chronic A handout on iron defi- blood cell production due to low iron inflammatory states, lead poisoning, thalas- ciency anemia, written by 1 the authors of this article, stores in the body. It is the most com- semia, and sideroblastic anemia. is available at http://www. mon nutritional disorder worldwide The following diagnostic approach is rec- aafp.org/afp/2013/0115/ I and accounts for approximately one-half of ommended in patients with anemia and is p98-s1.html. Access to anemia cases.1,2 Iron deficiency anemia can outlined in Figure 1.2,6-11 A serum ferritin level the handout is free and unrestricted. -

Medical Terminology Information Sheet
Medical Terminology Information Sheet: Medical Chart Organization: • Demographics and insurance • Flow sheets • Physician Orders Medical History Terms: • Visit notes • CC Chief Complaint of Patient • Laboratory results • HPI History of Present Illness • Radiology results • ROS Review of Systems • Consultant notes • PMHx Past Medical History • Other communications • PSHx Past Surgical History • SHx & FHx Social & Family History Types of Patient Encounter Notes: • Medications and medication allergies • History and Physical • NKDA = no known drug allergies o PE Physical Exam o Lab Laboratory Studies Physical Examination Terms: o Radiology • PE= Physical Exam y x-rays • (+) = present y CT and MRI scans • (-) = Ф = negative or absent y ultrasounds • nl = normal o Assessment- Dx (diagnosis) or • wnl = within normal limits DDx (differential diagnosis) if diagnosis is unclear o R/O = rule out (if diagnosis is Laboratory Terminology: unclear) • CBC = complete blood count o Plan- Further tests, • Chem 7 (or Chem 8, 14, 20) = consultations, treatment, chemistry panels of 7,8,14,or 20 recommendations chemistry tests • The “SOAP” Note • BMP = basic Metabolic Panel o S = Subjective (what the • CMP = complete Metabolic Panel patient tells you) • LFTs = liver function tests o O = Objective (info from PE, • ABG = arterial blood gas labs, radiology) • UA = urine analysis o A = Assessment (Dx and DDx) • HbA1C= diabetes blood test o P = Plan (treatment, further tests, etc.) • Discharge Summary o Narrative in format o Summarizes the events of a hospital stay -

IDSA/ATS Consensus Guidelines on The
SUPPLEMENT ARTICLE Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults Lionel A. Mandell,1,a Richard G. Wunderink,2,a Antonio Anzueto,3,4 John G. Bartlett,7 G. Douglas Campbell,8 Nathan C. Dean,9,10 Scott F. Dowell,11 Thomas M. File, Jr.12,13 Daniel M. Musher,5,6 Michael S. Niederman,14,15 Antonio Torres,16 and Cynthia G. Whitney11 1McMaster University Medical School, Hamilton, Ontario, Canada; 2Northwestern University Feinberg School of Medicine, Chicago, Illinois; 3University of Texas Health Science Center and 4South Texas Veterans Health Care System, San Antonio, and 5Michael E. DeBakey Veterans Affairs Medical Center and 6Baylor College of Medicine, Houston, Texas; 7Johns Hopkins University School of Medicine, Baltimore, Maryland; 8Division of Pulmonary, Critical Care, and Sleep Medicine, University of Mississippi School of Medicine, Jackson; 9Division of Pulmonary and Critical Care Medicine, LDS Hospital, and 10University of Utah, Salt Lake City, Utah; 11Centers for Disease Control and Prevention, Atlanta, Georgia; 12Northeastern Ohio Universities College of Medicine, Rootstown, and 13Summa Health System, Akron, Ohio; 14State University of New York at Stony Brook, Stony Brook, and 15Department of Medicine, Winthrop University Hospital, Mineola, New York; and 16Cap de Servei de Pneumologia i Alle`rgia Respirato`ria, Institut Clı´nic del To`rax, Hospital Clı´nic de Barcelona, Facultat de Medicina, Universitat de Barcelona, Institut d’Investigacions Biome`diques August Pi i Sunyer, CIBER CB06/06/0028, Barcelona, Spain. EXECUTIVE SUMMARY priate starting point for consultation by specialists. Substantial overlap exists among the patients whom Improving the care of adult patients with community- these guidelines address and those discussed in the re- acquired pneumonia (CAP) has been the focus of many cently published guidelines for health care–associated different organizations, and several have developed pneumonia (HCAP). -

Art Therapy Engage the Creative Power of Art for Clinical Assessment and Treatment
MA in Counseling – Art Therapy Engage the creative power of art for clinical assessment and treatment. In September 2018, the Commission on Accreditation of Allied Health Education Programs, the largest programmatic accreditor of health science professions in the U.S., granted Edinboro’s Master of Arts in Counseling – Art Therapy the nation’s first standard-compliant accreditation from the Accreditation Council for Art Therapy Education. The Art Therapy program is dedicated to achieving excellence in counseling, art therapy education, research, clinical and community service while fostering the highest ethical standards. The program integrates both counseling and visual arts practice, engaging the creative power of art for clinical assessment and treatment. In particular, we promote (1) scholarly research abilities and evidence-based clinical practice, (2) cross-cultural competency, and (3) technologies of media art. Overarching Mission Statement. The students and faculty of the Counseling Programs at Edinboro University of Pennsylvania are a diverse community of learners collaboratively engaged in research, scholarship, leadership and service. Faculty are committed to providing developmentally sound academic and clinical experiences to educate counselors to be effective leader-practitioners in a pluralistic society. Collectively, our mission is to prepare professional counselors who embody ethical behavior, provide services to enhance the mental health and well-being of families, groups, couples, and individuals, and advocate on behalf of both the counseling profession and those who are served. Programmatically, the mission of the art therapy concentration is to empower the artist and clinician within the art therapist through an accessible, inter-personal educational experience designed to meet the diverse needs of an evolving student population. -

What Is Problem-Solving Therapy?
What is Problem-Solving Therapy? Problem-solving therapy refers to a psychological treatment that helps to teach you to effectively manage the negative effects of stressful events that can occur in life. Such stressors can be rather large, such as getting a divorce, experiencing the death of a loved one, losing a job, or having a chronic medical illness like cancer or heart disease. Negative stress can also result from the accumulation of multiple “minor” occurrences, such as ongoing family problems, financial difficulties, constantly dealing with traffic jams, or tense relationships with co-workers or a boss. When such stressful problems either create psychological problems or exacerbate existing medical problems, such as coping with cancer or difficulties adhering to a medication regimen, problem-solving therapy may be of help, either as a sole intervention or in combination with other approaches. Problem-solving therapy can also help people who have more complex problems, such as “wanting to find one’s personal meaning of life.” You can work with your psychologist to best determine how problem-solving therapy can be of help to you. Problem-solving therapy has been found to be effective for a wide range of problems, including: • Major depressive disorder • Generalized anxiety disorder • Emotional distress • Suicidal ideation • Relationship difficulties • Certain personality disorders • Poor quality of life and emotional distress related to medical illness, such as cancer or diabetes Problem-solving therapy can provide training in adaptive problem-solving skills as a means of better resolving and/or coping with stressful problems. Such skills include: • Making effective decisions. • Generating creative means of dealing with problems.