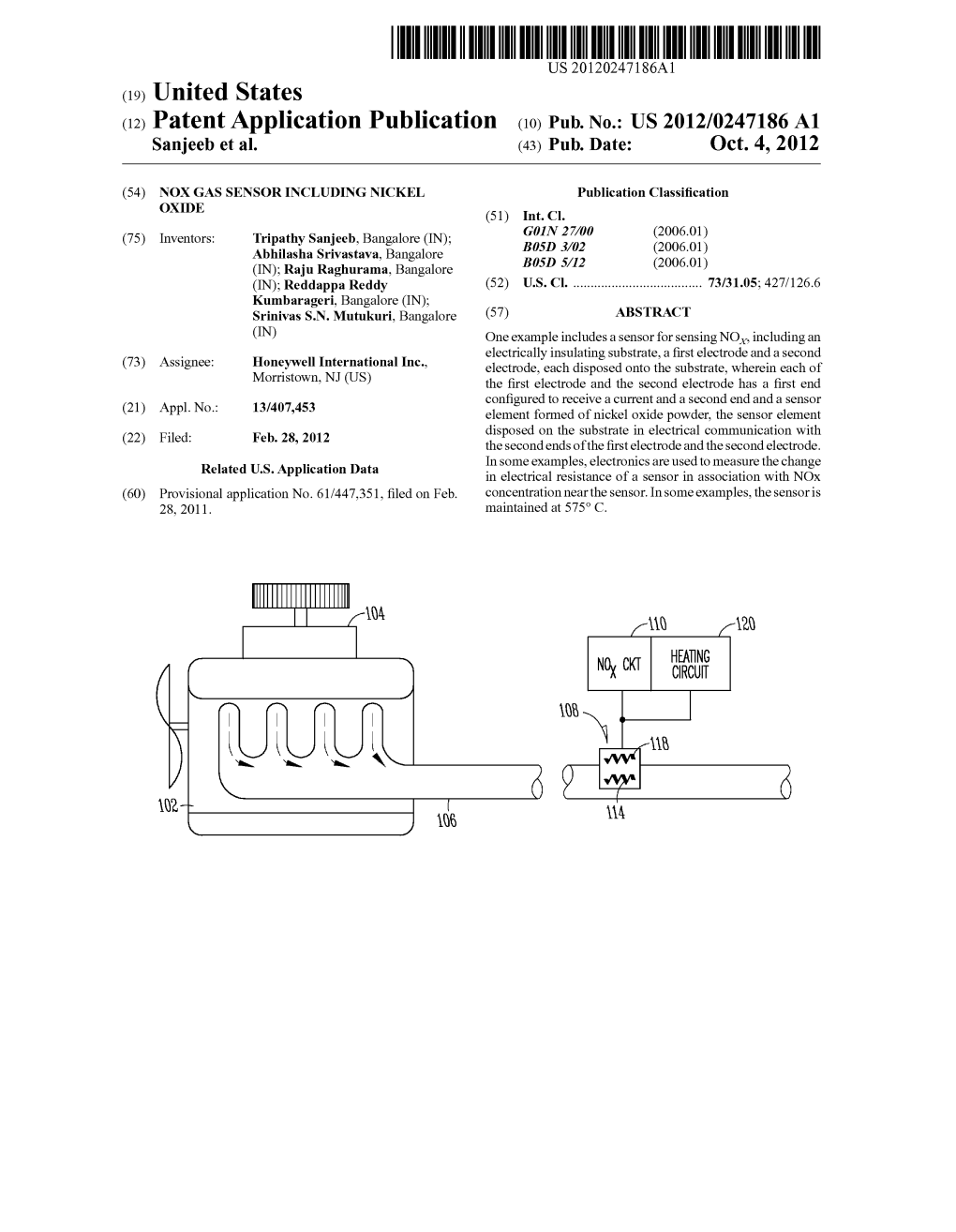
11111\Q /1111 0 9 “2” 0 W2 I 111 J 106 Patent Application Publication Oct
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wide Band Gap Materials and Devices for Nox, H2 and O2 Gas Sensing Applications
Wide band gap materials and devices for NOx, H2 and O2 gas sensing applications Dissertation zur Erlangung des akademischen Grades Doktor-Ingenieur (Dr.-Ing.) vorgelegt der Fakultät Elektrotechnik und Informationstechnik der Technischen Universität Ilmenau von Dipl.-Ing Majdeddin Ali geboren am 08.09.1976 in Homs, Syrien 1. Gutachter: Univ.-Prof. Dr. rer. nat. habil. Oliver Ambacher, Fraunhofer-Institut für Angewandte Festkörperphysik, Freiburg 2. Gutachter: PD Dr.-Ing. habil. Frank Schwierz, TU Ilmenau 3. Gutachter: Dr. Martin Eickhoff, Walter Schottky Institut, TU München Tag der Einreichung: 28.06.2007 Tag der wissenschaftlichen Aussprache: 22.01.2008 urn:nbn:de:gbv:ilm1-2007000323 Abstract III Abstract In this thesis, field effect gas sensors (Schottky diodes, MOS capacitors, and MOSFET transistors) based on wide band gap semiconductors like silicon carbide (SiC) and gallium nitride (GaN), as well as resistive gas sensors based on indium oxide (In2O3), have been developed for the detection of reducing gases (H2, D2) and oxidising gases (NOx, O2). The development of the sensors has been performed at the Institute for Micro- and Nanoelectronic, Technical University Ilmenau in co- operation with (GE) General Electric Global Research (USA) and Umwelt-Sensor- Technik GmbH (Geschwenda). Chapter 1: serves as an introduction into the scientific fields related to this work. The theoretical fundamentals of solid-state gas sensors are provided and the relevant properties of wide band gap materials (SiC and GaN) are summarized. In chapter 2: The performance of Pt/GaN Schottky diodes with different thickness of the catalytic metal were investigated as hydrogen gas detectors. The area as well as the thickness of the Pt were varied between 250 × 250 µm2 and 1000 × 1000 µm2, 8 and 40 nm, respectively. -

Diploma Thesis
CZECH TECHNICAL UNIVERSITY IN PRAGUE FACULTY OF ELECTRICAL ENGINEERING DIPLOMA THESIS Modeling and state estimation of automotive SCR catalyst JiˇríFigura Praha, 2016 Supervisor: Ing. Jaroslav Pekaˇr, Ph.D. Czech Technical University in Prague Faculty of Electrical Engineering Department of Control Engineering DIPLOMA THESIS ASSIGNMENT Student: Bc. Jiří Figura Study programme: Cybernetics and Robotics Specialisation: Systems and Control Title of Diploma Thesis: Modeling and state estimation of automotive SCR catalyst Guidelines: 1. Get introduced to automotive SCR and combined SCR/DPF systems 2. Provide an overview of the state-of-the-art in SCR state estimation and SCR modeling 3. Develop a model of automotive SCR catalyst for online estimation 4. Design an SCR estimator/observer of NH3 storage and analyze its performance Bibliography/Sources: [1] I. Nova and E. Tronconi, Eds., Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts. New York, NY: Springer New York, 2014 [2] C. Ericson, B. Westerberg, and I. Odenbrand, A state-space simplified SCR catalyst model for real time applications, SAE Technical Paper, 2008 [3] H. S. Surenahalli, G. Parker, and J. H. Johnson, Extended Kalman Filter Estimator for NH3 Storage, NO, NO2 and NH3 Estimation in a SCR, SAE International, Warrendale, PA, 2013-01-1581, Apr. 2013 [4] H. Zhang, J. Wang, and Y.-Y. Wang, Robust Filtering for Ammonia Coverage Estimation in Diesel Engine Selective Catalytic Reduction Systems, Journal of Dynamic Systems, Measurement, and Control, vol. 135, no. 6, pp. 064504-064504, Aug. 2013 Diploma Thesis Supervisor: Ing. Jaroslav Pekař, Ph.D. Valid until the summer semester 2016/2017 L.S. -

DTC P2200, P2202, P2203, P229E, P22A0, Or P22A1 • 2013 Chevrolet Silverado 4WD • Motologic
7/8/2020 DTC P2200, P2202, P2203, P229E, P22A0, or P22A1 • 2013 Chevrolet Silverado 4WD • MotoLogic DTC P2200, P2202, P2203, P229E, P22A0, or P22A1 Report a problem with this article Applies to: 6.6L (LGH or LML) Diagnostic Instructions Perform the Diagnostic System Check - Vehicle prior to using this diagnostic procedure. Review Strategy Based Diagnosis for an overview of the diagnostic approach. Diagnostic Procedure Instructions provides an overview of each diagnostic category. DTC Descriptors DTC P2200 NOx Sensor 1 Circuit DTC P229E NOx Sensor 2 Circuit DTC P2202 NOx Sensor 1 Circuit Low Voltage DTC P2203 NOx Sensor 1 Circuit High Voltage DTC P22A0 NOx Sensor 2 Circuit Low Voltage DTC P22A1 NOx Sensor 2 Circuit High Voltage Diagnostic Fault Information Short to Open/High Short to Signal Circuit Ground Resistance Voltage Performance U029D, U029D, U029E, U029D, U029E, NOx Sensor Ignition Voltage — P220A, U029E P220A, P220B P220B U0074, U010E, U0074, High Speed GMLAN Serial Data (+) — P205D P205D P205D U029D, U029E, U010E, U010E, High Speed GMLAN Serial Data (−) — U010E, P205D P205D P205D https://www.motologic.com/car/2013_chevrolet_silverado_4wd_3743/article/d2ee5c9d4549f5394cc56263b77426fb?returnPath=%2Fcar%2F2013_che… 1/5 7/8/2020 DTC P2200, P2202, P2203, P229E, P22A0, or P22A1 • 2013 Chevrolet Silverado 4WD • MotoLogic Short to Open/High Short to Signal Circuit Ground Resistance Voltage Performance U029D, NOx Sensor Ground — — — U029E Circuit/System Description The reductant system uses two nitrogen oxide (NOx) sensors to monitor the amount of NOx in the engine’s exhaust gas. The first sensor is located at the outlet of the turbocharger and monitors the engine out NOx level. The second NOx sensor is located between the selective catalytic reduction (SCR) and the diesel particulate filter (DPF) and monitors NOx levels downstream of the SCR. -

Nitrogen Oxides Gas Sensor Utilizing Microporous
DEVELOPMENT OF HARSH ENVIRONMENT NITROGEN OXIDES SOLID- STATE GAS SENSORS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Nicholas F. Szabo, M.S. ***** The Ohio State University 2003 Dissertation Committee: Approved by Dr. Prabir Dutta, Adviser Dr. Sheikh Akbar ______________________ Adviser Dr. Patrick Woodward Department of Chemistry ABSTRACT The goal of this dissertation was to study and develop high temperature solid-state sensors for combustion based gases. Specific attention was focused on NOx gases (NO and NO2) as they are of significant importance with respect to the environment and the health of living beings. This work is divided into four sections with the first chapter being an introduction into the effects of NOx gases and current regulations, followed by an introduction to the field of high temperature NOx sensors and finally where and why they will be needed in the future. Chapter 2 focuses on the development of a gas sensor for NOx capable of operation in harsh environments. The basis of the sensor is a mixed potential response at 500/600°C generated by exposure of gases to a platinum-yttria stabilized zirconia (Pt- YSZ) interface. Asymmetry between the two Pt electrodes on YSZ is generated by covering one of the electrodes with a zeolite, which helps to bring NO/NO2 towards equilibrium prior to the gases reaching the electrochemically active interface. Three sensor designs have been examined, including a planar design that is amenable to packaging for surviving automotive exhaust streams. Automotive tests indicated that the sensor is capable of detecting NO in engine exhausts. -

Nitrogen Oxide Sensor to Optimize Dispatchable Distributed Generation Systems
Energy Research and Development Division FINAL PROJECT REPORT Nitrogen Oxide Sensor to Optimize Dispatchable Distributed Generation Systems Gavin Newsom, Governor January 2021 | CEC-500-2021-006 PREPARED BY: Primary Authors: Ryan Ehlig Vince McDonell University of California, Irvine Irvine, CA 92697 Phone: 949-824-5950 | Fax: 949 824 7423 Contract Number: EPC-15-062 PREPARED FOR: California Energy Commission Yu Hou Project Manager Jonah Steinbuck, Ph.D Office Manager ENERGY GENERATION RESEARCH OFFICE Laurie ten Hope Deputy Director ENERGY RESEARCH AND DEVELOPMENT DIVISION Drew Bohan Executive Director DISCLAIMER This report was prepared as the result of work sponsored by the California Energy Commission. It does not necessarily represent the views of the Energy Commission, its employees or the State of California. The Energy Commission, the State of California, its employees, contractors and subcontractors make no warranty, express or implied, and assume no legal liability for the information in this report; nor does any party represent that the uses of this information will not infringe upon privately owned rights. This report has not been approved or disapproved by the California Energy Commission nor has the California Energy Commission passed upon the accuracy or adequacy of the information in this report. ACKNOWLEDGEMENTS The authors of this report would like to acknowledge Capstone Turbine Corporation for providing Combustion Lab at the University of California, Irvine (UCICL) with technical support for the C-60 Engine and support for modifying the engine software to implement the control logic developed. The authors would also like to acknowledge EmiSense and parent company CoorsTek for providing UCICL with the necessary sensors to complete this project report. -
(12) Patent Application Publication (10) Pub. No.: US 2015/0052975 A1
US 2015.0052975A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0052975 A1 Martin (43) Pub. Date: Feb. 26, 2015 (54) NETWORKED AIR QUALITY MONITORING (52) U.S. Cl. CPC ..... F24F II/0017 (2013.01); F24F 2011/0026 (71) Applicant: David Martin, Chagrin Falls, OH (US) (2013.01); F24F 2011/0027 (2013.01) (72) Inventor: David Martin, Chagrin Falls, OH (US) USPC ......................................................... 73/31.02 (21) Appl. No.: 14/533,305 (57) ABSTRACT (22) Filed: Nov. 5, 2014 Related U.S. Application Data Systems, methods, and non-transitory computer-readable (63) Continuation of application No. 13/737,102, filed on media for continuously monitoring residential air quality and Jan. 9, 2013, now Pat. No. 8,907,803. providing a trend based analysis regarding various air pollut (60) Provisional application No. 61/584,432, filed on Jan ants are presented herein. The system comprises an air quality 9, 2012 s - as monitor located in a residential house, wherein the air quality s monitor is configured to measure the level of an air pollutant. Publication Classification The system also includes a server that is communicatively coupled to the air quality monitor, wherein the server is con (51) Int. Cl. figured to generate a unique environmental fingerprint asso F24F II/00 (2006.01) ciated with the residential house. AIR QUALITY MONITOR 102 104 RAOO MODULE 100. / 106 SENSOR COMPONENT 108 SERVER 10 Patent Application Publication Feb. 26, 2015 Sheet 1 of 15 US 2015/0052975 A1 id 3 S g O 2. O e >- s C e s Patent Application Publication Feb. -
Dosimeter-Type Nox Sensing Properties of Kmno4 and Its Electrical Conductivity During Temperature Programmed Desorption
Dosimeter-Type NO[subscript x] Sensing Properties of KMnO[subscript 4] and Its Electrical Conductivity during Temperature Programmed Desorption The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Groß, Andrea, Michael Kremling, Isabella Marr, David Kubinski, Jacobus Visser, Harry Tuller, and Ralf Moos. “Dosimeter-Type NO[subscript x] Sensing Properties of KMnO[subscript 4] and Its Electrical Conductivity During Temperature Programmed Desorption.” Sensors 13, no. 4 (April 2013): 4428–4449. As Published http://dx.doi.org/10.3390/s130404428 Publisher MDPI AG Version Final published version Citable link http://hdl.handle.net/1721.1/92286 Terms of Use Creative Commons Attribution Detailed Terms http://creativecommons.org/licenses/by/3.0/ Sensors 2013, 13, 4428-4449; doi:10.3390/s130404428 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article Dosimeter-Type NOx Sensing Properties of KMnO4 and Its Electrical Conductivity during Temperature Programmed Desorption Andrea Groß 1, Michael Kremling 1, Isabella Marr 1, David J. Kubinski 2, Jacobus H. Visser 2, Harry L. Tuller 3 and Ralf Moos 1,* 1 Zentrum für Energietechnik, Bayreuth Engine Research Center (BERC), Department of Functional Materials, University of Bayreuth, 95440 Bayreuth, Germany; E-Mails: [email protected] (A.G.); [email protected] (M.K); [email protected] (I.M.) 2 Ford Research and Advanced Engineering, Dearborn, MI 48124, USA; E-Mail: [email protected] 3 Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-921-55-7401; Fax: +49-921-55-7405. -

SEMICONDUCTOR GAS SENSORS This Page Intentionally Left Blank Woodhead Publishing Series in Electronic and Optical Materials
SEMICONDUCTOR GAS SENSORS This page intentionally left blank Woodhead Publishing Series in Electronic and Optical Materials SEMICONDUCTOR GAS SENSORS Second Edition Edited by RAIVO JAANISO University of Tartu, Tartu, Estonia OOI KIANG TAN Nanyang Technological University, Singapore Woodhead Publishing is an imprint of Elsevier The Officers’ Mess Business Centre, Royston Road, Duxford, CB22 4QH, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, OX5 1GB, United Kingdom Copyright © 2020 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

Max Prüss" After Retrofitting with a SCRT System
Emission measurements on the laboratory vessel "Max Prüss" after retrofitting with a SCRT system Deliverable Part of Action B1, Emission reduction technologies The objective of LIFE CLINSH is to improve air quality in urban areas, situated close to ports and inland waterways, by accelerating IWT emission reductions. Project reference: LIFE15 ENV/NL/000217 Duration: 2016 – 2021 Project website: www.clinsh.eu CLINSH is an EC LIFE+ project delivered with the contribution of the LIFE financial instrument of the European Community. Project number LIFE15 ENV/NL/000217 This deliverable is part of Action B1, Emission reduction technologies. Contributors: LANUV NRW Version: End Date: 19.05.2020 Authors: Dieter Busch (LANUV NRW) Andreas Brandt (LANUV NRW) Martin Kleinebrahm (TÜV-Nord) Sergej Dreger (TÜV-Nord) CLINSH Deliverable: Part of Action B1, Emission reduction technologies 2 Emission measurements on the laboratory vessel "Max Prüss" after retrofitting with a SCRT system Project leader: Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV) Nordrhein-Westfalen Leibnizstrasse 10 45610 Recklinghausen Technical service: TÜV- Nord Mobilität GmbH & CO. KG Institut für Fahrzeugtechnik und Mobilität Adlerstrasse 7 45307 Essen Plant constructor: TEHAG Deutschland GmbH Gutenbergstrasse 42 47443 Moers Shipowner: Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV NRW) Nordrhein-Westfalen Leibnizstrasse 10 45610 Recklinghausen Cover photo: Eva Henneberger, Köln CLINSH Deliverable: Part of Action B1, Emission reduction technologies 3 Table of -
Elimination of Response to Relative Humidity Changes in Chemical- Sensitive Field-Effect Transistors † ‡ § † ‡ § † ‡ § ∥ ⊥ ∥ ⊥ Niharika Gupta, , , Hossain M
Article Cite This: ACS Sens. XXXX, XXX, XXX−XXX pubs.acs.org/acssensors Elimination of Response to Relative Humidity Changes in Chemical- Sensitive Field-Effect Transistors † ‡ § † ‡ § † ‡ § ∥ ⊥ ∥ ⊥ Niharika Gupta, , , Hossain M. Fahad, , , Matin Amani, , , Xiaohui Song, , Mary Scott, , † ‡ § and Ali Javey*, , , † ‡ ∥ Electrical Engineering & Computer Sciences, Berkeley Sensor and Actuator Center, and Department of Materials Science and Engineering, University of California, Berkeley, California 94720, United States § ⊥ Materials Sciences Division and The Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States *S Supporting Information ABSTRACT: Detecting accurate concentrations of gas in environments with dynamically changing relative humidity conditions has been a challenge in gas sensing technology. We report a method to eliminate effects of humidity response in chemical-sensitive field-effect transistors using microheaters. Using a hydrogen gas sensor with Pt/FOTS active material as a test case, we demonstrate that a sensor response of 3844% to a relative humidity change of 50 to 90% at 25 °C can be reduced to a negligible response of 11.6% by utilizing microheaters. We also show the advantage of this technique in maintaining the same sensitivity in changing ambient temperatures and its application to the nitrogen dioxide gas sensors. KEYWORDS: gas sensors, hydrogen, humidity, microheaters, temperature dependence electivity is one of the most important figures of merit for because of the complexity -

Development of an Ammonia Reduction After-Treatment Systems for Stoichiometric Natural Gas Engines
Graduate Theses, Dissertations, and Problem Reports 2017 Development of an Ammonia Reduction After-Treatment Systems for Stoichiometric Natural Gas Engines Saroj Pradhan Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Pradhan, Saroj, "Development of an Ammonia Reduction After-Treatment Systems for Stoichiometric Natural Gas Engines" (2017). Graduate Theses, Dissertations, and Problem Reports. 6447. https://researchrepository.wvu.edu/etd/6447 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Development of an Ammonia Reduction After-treatment Systems for Stoichiometric Natural Gas Engines Saroj Pradhan Dissertation submitted to the Benjamin M. Statler College of Engineering and Mineral Resources at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of -

Sensors in Iot Systems (Sense, Control and Actuate)
Sensors in IoT Systems (Sense, Control and Actuate) Introduction A typical IoT device contains a sensor for collecting information, signal processing for the output of sensor, digital logic for decision making and connectivity to internet and signal processing to actuate the actuator in response to that of sensed input. Analog Analog to Sensors Signal Digital processing Converter External Comm. Digital Logic Interface (BT, Wifi, etc) Digital to Driver / Actuator Analog Amplifier Converter Gateway A Typical IoT System Internet / Cloud PC / Cellphone Sensors in IoT System | Pradeep P | 6-Apr-2018 © 2018 Capgemini. All rights reserved. 2 Additional Requirements for IoT Sensors The basic functionality of Sensors is to sense and convert physical parameters into electrical signals. However, for IoT, sensors need to add the following properties too: • Low cost, to make IoT devices more economical for use in market • Small formfactor, so as to reduce size of IoT device and easy mounting in any environment • Wireless, for easy installation and to avoid issues with wired connectivity • Self-identification and self-validation, so it can alarm for it’s own failure • Very low power, for long lasting battery operation or manage with energy harvesting • Robust, to minimize or eliminate maintenance • Self-diagnostic and self-healing, detects own health • Self-calibrating, or accepts calibration commands via wireless link, for accurate results • Data pre-processing, to reduce load on gateways, PLCs and cloud resources In some of the applications single sensor may not be sufficient to do the job and hence multiple sensors can be combined and correlated to infer conclusions. For example, temperature sensor and vibration sensor data can be used to detect the onset of mechanical failure.