Global Characterization of Manganese Limitation in Bacillus Subtilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metatranscriptomic Analysis of Community Structure And
School of Environmental Sciences Metatranscriptomic analysis of community structure and metabolism of the rhizosphere microbiome by Thomas Richard Turner Submitted in partial fulfilment of the requirement for the degree of Doctor of Philosophy, September 2013 This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived there from must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. i Declaration I declare that this is an account of my own research and has not been submitted for a degree at any other university. The use of material from other sources has been properly and fully acknowledged, where appropriate. Thomas Richard Turner ii Acknowledgements I would like to thank my supervisors, Phil Poole and Alastair Grant, for their continued support and guidance over the past four years. I’m grateful to all members of my lab, both past and present, for advice and friendship. Graham Hood, I don’t know how we put up with each other, but I don’t think I could have done this without you. Cheers Salt! KK, thank you for all your help in the lab, and for Uma’s biryanis! Andrzej Tkatcz, thanks for the useful discussions about our projects. Alison East, thank you for all your support, particularly ensuring Graham and I did not kill each other. I’m grateful to Allan Downie and Colin Murrell for advice. For sequencing support, I’d like to thank TGAC, particularly Darren Heavens, Sophie Janacek, Kirsten McKlay and Melanie Febrer, as well as John Walshaw, Mark Alston and David Swarbreck for bioinformatic support. -

Protein Engineering of a Dye Decolorizing Peroxidase from Pleurotus Ostreatus for Efficient Lignocellulose Degradation
Protein Engineering of a Dye Decolorizing Peroxidase from Pleurotus ostreatus For Efficient Lignocellulose Degradation Abdulrahman Hirab Ali Alessa A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The University of Sheffield Faculty of Engineering Department of Chemical and Biological Engineering September 2018 ACKNOWLEDGEMENTS Firstly, I would like to express my profound gratitude to my parents, my wife, my sisters and brothers, for their continuous support and their unconditional love, without whom this would not be achieved. My thanks go to Tabuk University for sponsoring my PhD project. I would like to express my profound gratitude to Dr Wong for giving me the chance to undertake and complete my PhD project in his lab. Thank you for the continuous support and guidance throughout the past four years. I would also like to thank Dr Tee for invaluable scientific discussions and technical advices. Special thanks go to the former and current students in Wong’s research group without whom these four years would not be so special and exciting, Dr Pawel; Dr Hossam; Dr Zaki; Dr David Gonzales; Dr Inas,; Dr Yomi, Dr Miriam; Jose; Valeriane, Melvin, and Robert. ii SUMMARY Dye decolorizing peroxidases (DyPs) have received extensive attention due to their biotechnological importance and potential use in the biological treatment of lignocellulosic biomass. DyPs are haem-containing peroxidases which utilize hydrogen peroxide (H2O2) to catalyse the oxidation of a wide range of substrates. Similar to naturally occurring peroxidases, DyPs are not optimized for industrial utilization owing to their inactivation induced by excess amounts of H2O2. -

Biochemistry Centennial Celebration 1915 - 2015
BIOCHEMISTRY CENTENNIAL CELEBRATION 1915 - 2015 FEATURED SPEAKERS Dr. Hung-Ying Kao (Ph.D., 1995) Dr. Rebecca Moen (Ph.D., 2013) Professor of Biochemistry Assistant Professor of Chemistry & Geology Case Western Reserve University | Cleveland, OH Minnesota State University | Mankato, MN Dr. Venkateswarlu Pothapragada (Ph.D., 1962) Dr. Amy Rocklin (Ph.D., 2000) Division Scientist, 3M | Minneapolis-St. Paul, MN Corning, Inc. | Painted Post, NY Dr. Melanie Simpson (Ph.D., 1997) Dr. Brad Wallar (Ph.D., 2000) Professor of Biochemistry Associate Professor of Chemistry University of Nebraska | Lincoln, NE Grand Valley State University | Allendale, MI Thursday, May 14, 2015, 1:00-5:30 PM 2-470 Phillips-Wangensteen Building Minneapolis Campus Sponsored by The Frederick James Bollum Endowed Research Fund for Biochemistry NIVERSITY OF INNESOTA _____________________________________________________________________________________________U M Twin Cities Campus Department of Biochemistry, 6-155 Jackson Hall Molecular Biology and Biophysics 321 Church St. SE Minneapolis, MN, 55455 Medical School and V: (612) 625-6100 College of Biological Sciences F: (612) 625-2163 http://www.cbs.umn.edu/bmbb May 14, 2015 Dear Friends; Welcome to the Centennial Celebration commemorating the 100th anniversary of the first PhD granted in biochemistry at the University of Minnesota. Morris J. Blish was our first PhD recipient and he went on to a marvelously distinguished career in the food industry and was recognized by the U of MN in 1952 by President Morrill with the Outstanding -

Fatty Acid Metabolism Mediated by 12/15-Lipoxygenase Is a Novel Regulator of Hematopoietic Stem Cell Function and Myelopoiesis
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations Spring 2010 Fatty Acid Metabolism Mediated by 12/15-Lipoxygenase is a Novel Regulator of Hematopoietic Stem Cell Function and Myelopoiesis Michelle Kinder University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Immunology and Infectious Disease Commons Recommended Citation Kinder, Michelle, "Fatty Acid Metabolism Mediated by 12/15-Lipoxygenase is a Novel Regulator of Hematopoietic Stem Cell Function and Myelopoiesis" (2010). Publicly Accessible Penn Dissertations. 88. https://repository.upenn.edu/edissertations/88 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/88 For more information, please contact [email protected]. Fatty Acid Metabolism Mediated by 12/15-Lipoxygenase is a Novel Regulator of Hematopoietic Stem Cell Function and Myelopoiesis Abstract Fatty acid metabolism governs critical cellular processes in multiple cell types. The goal of my dissertation was to investigate the intersection between fatty acid metabolism and hematopoiesis. Although fatty acid metabolism has been extensively studied in mature hematopoietic subsets during inflammation, in developing hematopoietic cells the role of fatty acid metabolism, in particular by 12/ 15-Lipoxygenase (12/15-LOX), was unknown. The observation that 12/15-LOX-deficient (Alox15) mice developed a myeloid leukemia instigated my studies since leukemias are often a consequence of dysregulated hematopoiesis. This observation lead to the central hypothesis of this dissertation which is that polyunsaturated fatty acid metabolism mediated by 12/15-LOX participates in hematopoietic development. Using genetic mouse models and in vitro and in vivo cell development assays, I found that 12/15-LOX indeed regulates multiple stages of hematopoiesis including the function of hematopoietic stem cells (HSC) and the differentiation of B cells, T cells, basophils, granulocytes and monocytes. -

Chlorate Reduction
microorganisms Review Biotechnological Applications of Microbial (Per)chlorate Reduction Ouwei Wang 1,2 and John D. Coates 1,2,3,* 1 Department of Plant and Microbial Biology, University of California, Berkeley, CA 94720, USA; [email protected] 2 Energy Biosciences Institute, University of California, Berkeley, CA 94704, USA 3 Environmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA * Correspondence: [email protected] Received: 5 October 2017; Accepted: 22 November 2017; Published: 24 November 2017 Abstract: While the microbial degradation of a chloroxyanion-based herbicide was first observed nearly ninety years ago, only recently have researchers elucidated the underlying mechanisms of perchlorate and chlorate [collectively, (per)chlorate] respiration. Although the obvious application of these metabolisms lies in the bioremediation and attenuation of (per)chlorate in contaminated environments, a diversity of alternative and innovative biotechnological applications has been proposed based on the unique metabolic abilities of dissimilatory (per)chlorate-reducing bacteria (DPRB). This is fueled in part by the unique ability of these organisms to generate molecular oxygen as a transient intermediate of the central pathway of (per)chlorate respiration. This ability, along with other novel aspects of the metabolism, have resulted in a wide and disparate range of potential biotechnological applications being proposed, including enzymatic perchlorate detection; gas gangrene therapy; -

The Homopentameric Chlorite Dismutase from Magnetospirillum Sp
This item is the archived peer-reviewed author-version of: The homopentameric chlorite dismutase from Magnetospirillum sp. Reference: Freire Diana M., Rivas Maria G., Dias Andre M., Lopes Ana T., Costa Cristina, Santos-Silva Teresa, Van Doorslaer Sabine, Gonzalez Pablo J..- The homopentameric chlorite dismutase from Magnetospirillum sp. Journal of inorganic biochemistry - ISSN 0162-0134 - 151(2015), p. 1-9 Full text (Publishers DOI): http://dx.doi.org/doi:10.1016/j.jinorgbio.2015.07.006 To cite this reference: http://hdl.handle.net/10067/1310800151162165141 Institutional repository IRUA ÔØ ÅÒÙ×Ö ÔØ The Homopentameric Chlorite Dismutase from Magnetospirillum sp. Diana M. Freire, Maria G. Rivas, Andr´e M. Dias, Ana T. Lopes, Cristina Costa, Teresa Santos-Silva, Sabine Van Doorslaer, Pablo J. Gonz´alez PII: S0162-0134(15)30029-5 DOI: doi: 10.1016/j.jinorgbio.2015.07.006 Reference: JIB 9759 To appear in: Journal of Inorganic Biochemistry Received date: 18 February 2015 Revised date: 3 July 2015 Accepted date: 9 July 2015 Please cite this article as: Diana M. Freire, Maria G. Rivas, Andr´e M. Dias, Ana T. Lopes, Cristina Costa, Teresa Santos-Silva, Sabine Van Doorslaer, Pablo J. Gonz´alez, The Homopentameric Chlorite Dismutase from Magnetospirillum sp., Journal of Inorganic Biochemistry (2015), doi: 10.1016/j.jinorgbio.2015.07.006 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. -

A Meta-Analysis of the Effects of High-LET Ionizing Radiations in Human Gene Expression
Supplementary Materials A Meta-Analysis of the Effects of High-LET Ionizing Radiations in Human Gene Expression Table S1. Statistically significant DEGs (Adj. p-value < 0.01) derived from meta-analysis for samples irradiated with high doses of HZE particles, collected 6-24 h post-IR not common with any other meta- analysis group. This meta-analysis group consists of 3 DEG lists obtained from DGEA, using a total of 11 control and 11 irradiated samples [Data Series: E-MTAB-5761 and E-MTAB-5754]. Ensembl ID Gene Symbol Gene Description Up-Regulated Genes ↑ (2425) ENSG00000000938 FGR FGR proto-oncogene, Src family tyrosine kinase ENSG00000001036 FUCA2 alpha-L-fucosidase 2 ENSG00000001084 GCLC glutamate-cysteine ligase catalytic subunit ENSG00000001631 KRIT1 KRIT1 ankyrin repeat containing ENSG00000002079 MYH16 myosin heavy chain 16 pseudogene ENSG00000002587 HS3ST1 heparan sulfate-glucosamine 3-sulfotransferase 1 ENSG00000003056 M6PR mannose-6-phosphate receptor, cation dependent ENSG00000004059 ARF5 ADP ribosylation factor 5 ENSG00000004777 ARHGAP33 Rho GTPase activating protein 33 ENSG00000004799 PDK4 pyruvate dehydrogenase kinase 4 ENSG00000004848 ARX aristaless related homeobox ENSG00000005022 SLC25A5 solute carrier family 25 member 5 ENSG00000005108 THSD7A thrombospondin type 1 domain containing 7A ENSG00000005194 CIAPIN1 cytokine induced apoptosis inhibitor 1 ENSG00000005381 MPO myeloperoxidase ENSG00000005486 RHBDD2 rhomboid domain containing 2 ENSG00000005884 ITGA3 integrin subunit alpha 3 ENSG00000006016 CRLF1 cytokine receptor like -

Open Dissertation Rajakovichlj.Pdf
The Pennsylvania State University The Graduate School Eberly College of Science EXPLORING THE FUNCTIONAL AND MECHANISTIC DIVERSITY OF DIIRON OXIDASES AND OXYGENASES A Dissertation in Biochemistry, Microbiology, and Molecular Biology by Lauren J. Rajakovich 2017 Lauren J. Rajakovich Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2017 ii The dissertation of Lauren J. Rajakovich was reviewed and approved* by the following: J. Martin Bollinger, Jr. Professor of Chemistry Professor of Biochemistry and Molecular Biology Dissertation Co-Advisor Committee Co-Chair Carsten Krebs Professor of Chemistry Professor of Biochemistry and Molecular Biology Dissertation Co-Advisor Committee Co-Chair Squire J. Booker Howard Hughes Medical Investigator Professor of Chemistry Professor of Biochemistry and Molecular Biology Amie K. Boal Professor of Chemistry Professor of Biochemistry and Molecular Biology Christopher House Professor of Geosciences Scott Selleck Professor of Biochemistry and Molecular Biology Head of the Department of Biochemistry, Microbiology, and Molecular Biology *Signatures are on file in the Graduate School iii ABSTRACT Approximately half of all enzymes in Nature utilize a metal to perform their biological function. Many of the metalloenzymes that harbor transition metals activate dioxygen to catalyze a diverse array of oxidation reactions to functionalize unreactive sites in biomolecules. These enzymatic transformations are often inaccessible to synthetic chemists, and consequently, understanding the naturally-evolved mechanisms by which these enzymes enact such challenging reactions will enable the development of new biocatalysts for industrial and therapeutic applications. One common strategy employed in Nature is the coupling of two transition metals, typically iron, to carry out oxidation and oxygenation reactions. -
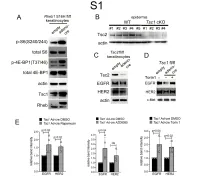
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test). -

All Enzymes in BRENDA™ the Comprehensive Enzyme Information System
All enzymes in BRENDA™ The Comprehensive Enzyme Information System http://www.brenda-enzymes.org/index.php4?page=information/all_enzymes.php4 1.1.1.1 alcohol dehydrogenase 1.1.1.B1 D-arabitol-phosphate dehydrogenase 1.1.1.2 alcohol dehydrogenase (NADP+) 1.1.1.B3 (S)-specific secondary alcohol dehydrogenase 1.1.1.3 homoserine dehydrogenase 1.1.1.B4 (R)-specific secondary alcohol dehydrogenase 1.1.1.4 (R,R)-butanediol dehydrogenase 1.1.1.5 acetoin dehydrogenase 1.1.1.B5 NADP-retinol dehydrogenase 1.1.1.6 glycerol dehydrogenase 1.1.1.7 propanediol-phosphate dehydrogenase 1.1.1.8 glycerol-3-phosphate dehydrogenase (NAD+) 1.1.1.9 D-xylulose reductase 1.1.1.10 L-xylulose reductase 1.1.1.11 D-arabinitol 4-dehydrogenase 1.1.1.12 L-arabinitol 4-dehydrogenase 1.1.1.13 L-arabinitol 2-dehydrogenase 1.1.1.14 L-iditol 2-dehydrogenase 1.1.1.15 D-iditol 2-dehydrogenase 1.1.1.16 galactitol 2-dehydrogenase 1.1.1.17 mannitol-1-phosphate 5-dehydrogenase 1.1.1.18 inositol 2-dehydrogenase 1.1.1.19 glucuronate reductase 1.1.1.20 glucuronolactone reductase 1.1.1.21 aldehyde reductase 1.1.1.22 UDP-glucose 6-dehydrogenase 1.1.1.23 histidinol dehydrogenase 1.1.1.24 quinate dehydrogenase 1.1.1.25 shikimate dehydrogenase 1.1.1.26 glyoxylate reductase 1.1.1.27 L-lactate dehydrogenase 1.1.1.28 D-lactate dehydrogenase 1.1.1.29 glycerate dehydrogenase 1.1.1.30 3-hydroxybutyrate dehydrogenase 1.1.1.31 3-hydroxyisobutyrate dehydrogenase 1.1.1.32 mevaldate reductase 1.1.1.33 mevaldate reductase (NADPH) 1.1.1.34 hydroxymethylglutaryl-CoA reductase (NADPH) 1.1.1.35 3-hydroxyacyl-CoA -

Science-2013-Meijer-82-5.Pdf
Translational Repression and eIF4A2 Activity Are Critical for MicroRNA-Mediated Gene Regulation H. A. Meijer et al. Science 340, 82 (2013); DOI: 10.1126/science.1231197 This copy is for your personal, non-commercial use only. If you wish to distribute this article to others, you can order high-quality copies for your colleagues, clients, or customers by clicking here. Permission to republish or repurpose articles or portions of articles can be obtained by following the guidelines here. The following resources related to this article are available online at www.sciencemag.org (this information is current as of April 9, 2013 ): Updated information and services, including high-resolution figures, can be found in the online on April 9, 2013 version of this article at: http://www.sciencemag.org/content/340/6128/82.full.html Supporting Online Material can be found at: http://www.sciencemag.org/content/suppl/2013/04/03/340.6128.82.DC1.html This article cites 38 articles, 25 of which can be accessed free: http://www.sciencemag.org/content/340/6128/82.full.html#ref-list-1 This article appears in the following subject collections: www.sciencemag.org Molecular Biology http://www.sciencemag.org/cgi/collection/molec_biol Downloaded from Science (print ISSN 0036-8075; online ISSN 1095-9203) is published weekly, except the last week in December, by the American Association for the Advancement of Science, 1200 New York Avenue NW, Washington, DC 20005. Copyright 2013 by the American Association for the Advancement of Science; all rights reserved. The title Science is a registered trademark of AAAS. -

Transiently Produced Hypochlorite Is Responsible for the Irreversible Inhibition of Chlorite Dismutase † † † ‡ † Stefan Hofbauer, Clemens Gruber, Katharina F
Article pubs.acs.org/biochemistry Terms of Use CC-BY Transiently Produced Hypochlorite Is Responsible for the Irreversible Inhibition of Chlorite Dismutase † † † ‡ † Stefan Hofbauer, Clemens Gruber, Katharina F. Pirker, Axel Sündermann, Irene Schaffner, † ‡ † † Christa Jakopitsch, Chris Oostenbrink, Paul G. Furtmüller, and Christian Obinger*, † Department of Chemistry, Division of Biochemistry, VIBT-Vienna Institute of BioTechnology, BOKU-University of Natural Resources and Life Sciences, A-1190 Vienna, Austria ‡ Department of Material Sciences and Process Engineering, Institute of Molecular Modeling and Simulation, BOKU-University of Natural Resources and Life Sciences, A-1190 Vienna, Austria *S Supporting Information ABSTRACT: Chlorite dismutases (Clds) are heme b-containing prokaryotic oxidoreductases that catalyze the reduction of chlorite to chloride with the concomitant release of molecular oxygen. Over time, they are irreversibly inactivated. To elucidate the mechanism of inactivation and investigate the role of the postulated intermediate hypochlorite, the pentameric chlorite dismutase of “Candidatus Nitrospira defluvii” (NdCld) and two variants (having the conserved distal arginine 173 exchanged with alanine and lysine) were recombinantly produced in Escherichia coli. Exchange of the distal arginine boosts the extent of irreversible inactivation. In the presence of the hypochlorite traps methionine, monochlorodimedone, and 2-[6-(4- aminophenoxy)-3-oxo-3H-xanthen-9-yl]benzoic acid, the extent of chlorite degradation and release of molecular oxygen is significantly increased, whereas heme bleaching and oxidative modifications of the protein are suppressed. Among other modifications, hypochlorite-mediated formation of chlorinated tyrosines is demonstrated by mass spectrometry. The data obtained were analyzed with respect to the proposed reaction mechanism for chlorite degradation and its dependence on pH.