Amy Deacon Phd Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FAMILY Poeciliidae Bonaparte 1831
FAMILY Poeciliidae Bonaparte 1831 - viviparous toothcarps, livebearers SUBFAMILY Poeciliinae Bonaparte 1831 - viviparous toothcarps [=Unipupillati, Paecilini, Belonesocini, Cyprinodontidae limnophagae, Gambusiinae, Tomeurinae, Poeciliopsinae, Heterandriini, Guirardinini, Cnesterodontini, Pamphoriini, Xiphophorini, Alfarini, Quintanini, Xenodexiinae, Dicerophallini, Scolichthyinae, Priapellini, Brachyrhaphini, Priapichthyini] GENUS Alfaro Meek, 1912 - livebearers [=Furcipenis, Petalosoma, Petalurichthys] Species Alfaro cultratus (Regan, 1908) - Regan's alfaro [=acutiventralis, amazonum] Species Alfaro huberi (Fowler, 1923) - Fowler's alfaro GENUS Belonesox Kner, 1860 - pike topminnows Species Belonesox belizanus Kner, 1860 - pike topminnow [=maxillosus] GENUS Brachyrhaphis Regan, 1913 - viviparous toothcarps [=Plectrophallus, Trigonophallus] Species Brachyrhaphis cascajalensis (Meek & Hildebrand, 1913) - Río Cascajal toothcarp Species Brachyrhaphis episcopi (Steindachner, 1878) - Obispo toothcarp [=latipunctata] Species Brachyrhaphis hartwegi Rosen & Bailey, 1963 - Soconusco gambusia Species Brachyrhaphis hessfeldi Meyer & Etzel, 2001 - Palenque toothcarp Species Brachyrhaphis holdridgei Bussing, 1967 - Tronadora toothcarp Species Brachyrhaphis olomina (Meek, 1914) - Orotina toothcarp Species Brachyrhaphis parismina (Meek, 1912) - Parismina toothcarp Species Brachyrhaphis punctifer (Hubbs, 1926) - Quibari Creek toothcarp Species Brachyrhaphis rhabdophora (Regan, 1908) - Río Grande de Terraba toothcarp [=tristani] Species Brachyrhaphis roseni -

A REVISION of the GAMBUSIA NICARAGUENSIS SPECIES GROUP (PISCES:POECILIIDAE) by William L. Fink ABSTRACT in Addition to Gambusia
Reprinted from PUBLICATIONS OF THE GULF COAST RESEARCH LABORATORY MUSEUM 2:47-77, June 18, 1971 A REVISION OF THE GAMBUSIA NICARAGUENSIS SPECIES GROUP (PISCES:POECILIIDAE) by William L. Fink ABSTRACT In addition to Gambusia nicaraguensis, the species group includes G. wrayi, G. mela pleura and G. his paniolae sp. nov. G. gracilior is a junior synonym of G. wrayi and G. dominicensis is found to be a member of another species group. A key and zoogeographical notes are provided for the group. Rivas (1963) published on subgenera and species groups in the genus Gambusia. He used only gonopodial characters in defining his groups, and I believe that his system is both natural and practical. Subsequent investigation has shown a need to review his findings and to make adjust- ments in the system. I have found that G. dominicensis is a member of another species group and that the species referred to as dominicensis by Rivas (1963) is actually undescribed. Otherwise, I accept his G. nicara- guensis species group and feel that its revision will help clarify other prob- lems within the genus. METHODS.—Methods are those of Fink (1971). Abbreviations are as follows: ANSP - Academy of Natural Sciences of Philadelphia; BMNH - British Museum (Natural History); GCRL - Gulf Coast Re- search Laboratory; UMMZ - University of Michigan Museum of Zoology; USNM - United States National Museum. Unless otherwise noted, lengths are standard length (SL); descriptions of coloration are from alcoholic specimens; all material examined is not included in the tables. 47 DIAGNOSIS OF THE SPECIES GROUP.—Length of gonopodium about one-third of SL. -
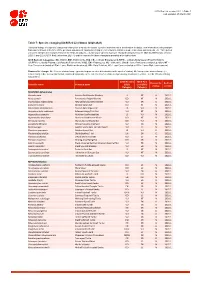
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Publications of the Gulf Coast Research Laboratory Museum, Vol. 2 C.E
The University of Southern Mississippi The Aquila Digital Community GCRL Publications 6-18-1971 Publications of the Gulf Coast Research Laboratory Museum, Vol. 2 C.E. Dawson Gulf Coast Research Laboratory Follow this and additional works at: https://aquila.usm.edu/gcrl_publications Recommended Citation Dawson, C.E., "Publications of the Gulf Coast Research Laboratory Museum, Vol. 2" (1971). GCRL Publications. 1. https://aquila.usm.edu/gcrl_publications/1 This Book is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in GCRL Publications by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. Publications of the Gulf Coast Research Laboratory Museum I 2 Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 Published June 18, 1971 l Publications of the Gulf Coast Research Laboratory Museum 2 Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 Published June 18, 1971 Published by Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 U.S. A. C. E. Dawson, Editor T his publication may be obtained from the Laboratory Bookstore at a price of $1.25 per copy. Wilkes Printing Company, Inc., Biloxi, Mississippi TABLE OF CONTENTS Ri vas, Lu is R. A new subspecies of poeciliid f ishes of the genus Gambusia f rom eastern Cuba ----------------------------------- 5 Fi nk, Willia m L. A revision of the Gambusia puncticulata complex (Pi sees: Poec il i i d ae) _________________ ------------------------------ l l Fi nk, William L. A revision of the Gambusia nicaraguensis species g roup (P isces: Poeci I i idae) ______________ ·-------------------·---- 47 3 A NEW SUBSPECIES OF POECILIID FISHES OF THE GENUS GAMBUSIA FROM EASTERN CUBA by Luis R. -

Priocharax Nanus, a New Miniature Characid from the Rio Negro
Neotropical Ichthyology, 12(2): 229-246, 2014 Copyright © 2014 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20130171 Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes Mônica Toledo-Piza1, George M. T. Mattox2 and Ralf Britz3 Priocharax nanus, new species, is described from the rio Negro, Brazil. It is a miniature fish that retains as an adult the larval rayless pectoral fin, a diagnostic character of the genus. Priocharax nanus possesses fewer reductive features compared to congeners, P. ariel and P. pygmaeus, from which it can be distinguished by the presence of i,6 pelvic-fin rays (vs. i,5), the presence of the claustrum (vs. claustrum absent) and the presence of two postcleithra (vs. postcleithra absent). An updated list of 213 species of miniature Neotropical freshwater fishes is presented. The greatest diversity among them is represented by the Characiformes with 87 miniature species. Priocharax nanus, espécie nova, é descrita do rio Negro, Brasil. É um peixe miniatura que retém no adulto a forma larval da nadadeira peitoral, um caráter diagnóstico do gênero. Priocharax nanus possui um número menor de caracteres redutivos quando comparado aos congêneres, P. ariel and P. pygmaeus, dos quais pode ser distinguida pela presença de i,6 raios na nadadeira pélvica (vs. i,5), presença do claustrum (vs. claustrum ausente) e presença de dois pós-cleitros (vs. pós-cleitros ausentes). Uma lista atualizada de 213 espécies de peixes miniatura de água doce neotropicais é apresentada. A maior diversidade entre eles é representada pelos Characiformes, com 87 espécies miniatura. -

View/Download
CYPRINODONTIFORMES (part 4) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 11.0 - 11 April 2021 Order CYPRINODONTIFORMES (part 4 of 4) Suborder CYPRINODONTOIDEI (cont.) Family POECILIIDAE Poeciliids 39 genera/subgenera · 281 species/subspecies Subfamily Xenodexiinae Grijalva Studfish Xenodexia Hubbs 1950 xenos, strange; dexia, right hand, referring to axillary region of right pectoral fin “spectacularly modified” into a sort of “clasper” with an “assortment of hooks, pads, and other processes” (the precise copulatory function of this “clasper” remains unknown) Xenodexia ctenolepis Hubbs 1950 ctenos, comb; lepis, scale, referring to its ctenoid scales, unique in Cyprinodontiformes Subfamily Tomeurinae Tomeurus Eigenmann 1909 tomeus, knife; oura, tail, referring to ventral “knife-like” ridge, resembling an adipose fin but composed of ~16 paired scales, extending almost entire length of caudal peduncle Tomeurus gracilis Eigenmann 1909 slender, described as “Very long and slender” Subfamily Poeciliinae Livebearers Alfaro Meek 1912 named for Anastasio Alfaro (1865-1951), archaeologist, geologist, ethnologist, zoologist, Director of the National Museum of Costa Rica (type locality of A. cultratus), and “the best known scientist of the Republic” Alfaro cultratus (Regan 1908) knife-shaped, referring to lower surface of tail compressed to a sharp edge Alfaro huberi (Fowler 1923) in honor of Wharton Huber (1877-1942), Curator of Mammals, Academy of Natural Sciences of Philadelphia (where Fowler worked), who collected type Belonesox Kner 1860 resembling both the needlefish, Belone, and the pike, Esox Belonesox belizanus belizanus Kner 1860 -anus, belonging to: Belize, type locality (also occurs in Costa Rica, Honduras, México and Nicaragua) Belonesox belizanus maxillosus Hubbs 1936 pertaining to the jaw, referring to its “very heavy jaws” Brachyrhaphis Regan 1913 brachy, short; rhaphis, needle, presumably referring to shorter gonopodium compared to Gambusia, original genus of type species, B. -

Review of Impacts of Displaced/Introduced Fauna Associated with Inland Waters
Review of Impacts of Displaced/Introduced Fauna Associated with Inland Waters Author Arthington, Angela, McKenzie, Fiona Published 1998 Copyright Statement © Commonwealth of Australia 1997. This work is copyright. It may be reproduced in whole or in part for study, research or training purposes subject to the inclusion of an acknowledgment of the source and no commercial usage or sale. Reproduction for purposes other than those listed above requires the written permission of the Australian Government Publishing Service. Downloaded from http://hdl.handle.net/10072/181935 Griffith Research Online https://research-repository.griffith.edu.au Review of Impacts of Displaced/Introduced Fauna Associated with Inland Waters Angela Arthington Fiona McKenzie Centre for Catchment and In-stream Research Griffith University Nathan Qld 4111 Australia: State of the Environment Technical Paper Series (Inland Waters) Environment Australia, part of the Department of the Environment E Commonwealth of Australia 1997 This work is copyright. It may be reproduced in whole or in part for study, research or training purposes subject to the inclusion of an acknowledgment of the source and no commercial usage or sale. Reproduction for purposes other than those listed above requires the written permission of the Australian Government Publishing Service. Requests and enquiries concerning reproduction and rights should be addressed to the Manager, Commonwealth Information Services, AGPS, GPO Box 84, Canberra ACT 2601, Australia. The Commonwealth accepts no responsibility for the opinions expressed in this document, or the accuracy or completeness of the contents of this document. The Commonwealth will not be liable for any loss or damage occasioned directly or indirectly through the use of, or reliance on, the contents of this document. -
Gambusia Dominicensis) Ecological Risk Screening Summary
Dominican Gambusia (Gambusia dominicensis) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2011 Revised, September 2018 Web Version, 6/5/2019 Image: Regan (1913). Public domain. Available: https://www.biodiversitylibrary.org/page/31977331#page/814/mode/1up. (September 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Central America: Haiti and Dominican Republic.” From Snoeks et al. (2009): “This species' native range is Lac Azuei in Haiti (111.6 km²) and Lago Enriquillo (259.5 km²) in the Dominican Republic.” Status in the United States This species has not been reported as introduced or established in the United States. There is no indication that this species is in trade in the United States. 1 Means of Introductions in the United States This species has not been reported as introduced or established in the United States. Remarks From Snoeks et al. (2009): “Red List Category & Criteria: Endangered […]” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2018): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Acanthopterygii Order Cyprinodontiformes Suborder Cyprinodontoidei Family Poeciliidae Subfamily Poeciliinae Genus Gambusia Species Gambusia dominicensis Regan, 1913” From Fricke et al. (2018): “Current status: Valid as Gambusia dominicensis Regan 1913. Poeciliidae: Poeciliinae.” Size, Weight, and Age Range From Froese and Pauly (2018): “Max length : 2.5 cm TL male/unsexed; [Merrick and Schmida 1984]; 6.0 cm (female)” Environment From Froese and Pauly (2018): “Freshwater; brackish; benthopelagic; non-migratory. […] 22°C - 28°C” 2 Climate/Range From Froese and Pauly (2018): “Tropical; […]” Distribution Outside the United States Native From Froese and Pauly (2018): “Central America: Haiti and Dominican Republic.” From Snoeks et al. -

CARES Exchange March 2017
The CARES Exchange Volume I Number I CARES Area of Concern: Lake Victoria March 2017 The Directory of Available CARES Species New CARES Website Launch Meet Your CARES Team Calendar of Aquatic Events 2 Welcome to the charter issue of The CARES Ex- If your organization is interested in participating in change. The primary intent of this publication is to CARES, review the ‘CARES Startup’ tab on the web- make available a listing of CARES fish from the CA- site CARESforfish.org, then contact Klaus Steinhaus RES membership to those that may be searching for at CARES species. [email protected]. ___________________________________________ It is important to understand that all transactions are Species maintenance is serious business. When deal- between the buyer and seller and CARES in no way ing with CARES Priority Listed species, you are liter- moderates any exchanges including shipping prob- ally the guardian of that animal. Yes it is a collective lems, refunds, or bad blood between the two parties. effort but one not to be taken lightly. When contact- This directory merely provides an avenue to which ing a CARES availability list participant to obtain a CARES fish may be located. As with all sales, be cer- fish, please ensure you have done ample research to tain that all the elements of the exchange are worked ensure you can provide a suitable environment for its out before purchasing or shipping. long-term care. Many questions can be answered on the CARES Facebook group at https:// No hybrids will knowingly be listed. www.facebook.com/groups/153148624721520/. -

Poe494 2006.Pdf
Poeyana 494:1-30 Información bibliográfíca sobre los peces dulceacuícolas de Las Antillas* Ignacio RAMOS GARCÍA** ABSTRACT: Bibliographical information of The Antillean freshwater fishes. A list of published works on the Antillean freshwater fish fauna, alphabetically indexed by authors and dates, is offered. To facilitate searches; authors, species, geographical and topic indexes are also included. A list of the Antillean freshwater fish fauna by countries is also provided. KEY WORDS: Freshwater fishes, References, Species list, West Indies. INTRODUCCIÓN se dan los siguientes índices: por autores; por especies, en el caso de tener la información se da entre paréntesis las Cuando nos enfrentamos al estudio o investigación de un sinonimias y el nombre vulgar; geográfico y temático. En tema específico se necesita conocer el grado de conocimiento algunas referencias puede faltar el nombre del autor, el título existente hasta ese momento del asunto tratado, por lo que se del trabajo o las páginas; esta ausencia esta identificada con hace necesario y obligatorio realizar una búsqueda exhaustiva signos de interrogación (¿?), a pesar de faltar esta información de los trabajos relacionados con el tema realizados con decidimos poner estas fichas porque pensamos puedan ser de anterioridad. La intención de este trabajo es facilitar la utilidad. La información procesada para la confección de los búsqueda y recuperación de estos documentos para ser índices no fue homogénea, ya que en ocasiones no se pudo consultados por los interesados, ya sean aficionados o revisar la publicación y se procesó sólo por la información investigadores. Conocemos de la existencia de tres que brinda el título y por la inferida de las citas de otros publicaciones anteriores realizadas con este fin, pero dirigidas trabajos. -
Emerging Issues in Alien Fish Ma Nagement in the Murray-Darling Basin
s R KNOWLEDGE Rive EmErging issuEs in AliEn Fish mAnAgEmEnt in thE murrAy-DArling BAsin stAtEmEnt, rECOmmEnDAtiOns AnD suPPOrting PAPErs WOrKshOP hElD in BrisBAnE, 30-31 mAy 2006 EmErging issuEs in AliEn Fish mAnAgEmEnt in thE murrAy-DArling BAsin stAtEmEnt, rECOmmEndatiOns AnD suPPOrting papers WOrKshOP hElD in BrisBAnE, 30-31 mAy 2006 Dean AnsEll AnD PEtEr JacksOn (EDitOrs) Acknowledgements sincere thanks are extended to those who participated in the workshop. A special thanks to Peter Jackson, Peter Kind, natalie Baker, Jim Barrett, mark lintermans and Craig Boys for their efforts in organising and running the event. the workshop was expertly facilitated by Derek Foster. John Koehn and mark lintermans assisted greatly in preparation of the statement and recommendations for the proceedings. JunE 2007 Published by murray-Darling Basin Commission Postal Address GPO Box 409, Canberra ACt 2601 Office location Level 5, 15 moore street, Canberra City, Australian Capital territory telephone (02) 6279 0100 international + 61 2 6279 0100 Facsimile (02) 6248 8053 international + 61 2 6248 8053 E-mail [email protected] internet http://www.mdbc.gov.au For further information contact the murray-Darling Basin Commission office on (02) 6279 0100 this report may be cited as: Ansell, D. and Jackson, P. (Eds). 2007. Emerging Issues in Alien Fish Management in the Murray-Darling Basin: Statement, recommendations and supporting papers. Proceedings of a workshop held in Brisbane QlD, 30-31 may 2006. murray-Darling Basin Commission, Canberra. mDBC Publication no: 16/07 graphic design by Art Direction Creative, manuka ACt isBn: 1 921257 26 1 © Copyright murray-Darling Basin Commission 2007 this work is copyright. -

Developing Tilapia Aquaculture in Haiti: Opportunities, Constraints, and Action Items
DEVELOPING TILAPIA AQUACULTURE IN HAITI: OPPORTUNITIES, CONSTRAINTS, AND ACTION ITEMS PROCEEDINGS OF A WORKSHOP SPONSORED BY NOVUS INTERNATIONAL, AQUACULTURE WITHOUT FRONTIERS, THE WORLD AQUACULTURE SOCIETY, AND THE MARINE BIOLOGICAL LABORATORY Edited by John A. Hargreaves Aquaculture Assessments LLC Reviewed by Craig Browdy Bill Mebane Dave Conley Valentin Abe Novus International Marine Biological Aquaculture Without Caribbean Harvest, Laboratory Frontiers Haiti 13 November 2012 FOREWARD After the devastating earthquake in 2010, informal discussions were held about potential forms of a post-crisis engagement with the part of the recovery and redevelopment process that involved aquaculture. This document represents the synthesis of discussions held at a consultative meeting organized and supported by Novus International, the Marine Biological Laboratory at Woods Hole, Aquaculture without Frontiers, and the World Aquaculture Society. Around 50 representatives participated in a one-day facilitated meeting in New Orleans, LA, in February, 2011. The workshop brought together three groups interested in earthquake recovery and aquaculture development of the country: 1) Haitians working on the ground, including hatchery operators, tilapia growers, government officials, individuals and groups implementing projects for NGOs, and entrepreneurs; 2) generalists working for small to large, usually faith-based NGOs with a diverse portfolio of activities, including but not emphasizing small-scale aquaculture; 3) experienced scientists and commercial practitioners representing best practices and collective wisdom. To provide context, presentations were made about the current situation with aquaculture in Haiti. Then the group identified and discussed 1) models with the best potential for commercial development, 2) regions with the most suitable sites for ponds, 3) opportunities and constraints to aquaculture development, and 4) prioritized items for action.