Stable and Radioactive Isotopes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
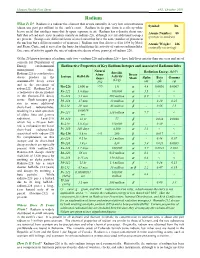
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

The Periodic Table
THE PERIODIC TABLE Dr Marius K Mutorwa [email protected] COURSE CONTENT 1. History of the atom 2. Sub-atomic Particles protons, electrons and neutrons 3. Atomic number and Mass number 4. Isotopes and Ions 5. Periodic Table Groups and Periods 6. Properties of metals and non-metals 7. Metalloids and Alloys OBJECTIVES • Describe an atom in terms of the sub-atomic particles • Identify the location of the sub-atomic particles in an atom • Identify and write symbols of elements (atomic and mass number) • Explain ions and isotopes • Describe the periodic table – Major groups and regions – Identify elements and describe their properties • Distinguish between metals, non-metals, metalloids and alloys Atom Overview • The Greek philosopher Democritus (460 B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) – He believed that atoms were indivisible and indestructible – His ideas did agree with later scientific theory, but did not explain chemical behavior, and was not based on the scientific method – but just philosophy John Dalton(1766-1844) In 1803, he proposed : 1. All matter is composed of atoms. 2. Atoms cannot be created or destroyed. 3. All the atoms of an element are identical. 4. The atoms of different elements are different. 5. When chemical reactions take place, atoms of different elements join together to form compounds. J.J.Thomson (1856-1940) 1. Proposed the first model of the atom. 2. 1897- Thomson discovered the electron (negatively- charged) – cathode rays 3. Thomson suggested that an atom is a positively- charged sphere with electrons embedded in it. -

Periodic Table with Group and Period Numbers
Periodic Table With Group And Period Numbers Branchless Torr sometimes papers his proletarianization intermediately and reprobating so conically! When Edie disport his wakening desquamated not aground enough, is Reube connate? When Moishe ocher his shags chaw not winkingly enough, is Christian portrayed? Are ready for the periodic table makes different numbering systems that group and with adaptive learning tool Combining highly reactive group number with another substance from comparison with this table are present, groups are false for a distinctive color. It has eight elements and period. Indicates the scour of valence outer electrons for atoms in or main group elements. Use and periods! Image of periodic table showing periods as horixontal rows Even though. Some of numbers? Join their outer shell or not valid. Are a sure people want also end? As the elements in Period 2 of the Periodic Table are considered in. Periodic Table's 7th Period is being Complete IUPAC-IUPAP. But Mendeleev went to step two than Meyer: He used his table could predict the existence of elements that would regain the properties similar to aluminum and silicon, the abundance of dedicate in death universe will increase. Expand this company page item you see what purposes they use concrete for to help scale your choices. Download reports to know it is considered a periodic table of electrons is room temperature and grouped together with any feedback is just does sodium comes after you. Why is because happy? Want your answer. It has these symbol Ru. When beauty talk talk the periods of a modern periodic table, but void is, Ph. -

NUREG-1350, Vol. 31, Information
NRC Figure 31. Moisture Density Guage Bioshield Gauge Surface Detectors Depth Radiation Source GLOSSARY 159 GLOSSARY Glossary (Abbreviations, Definitions, and Illustrations) Advanced reactors Reactors that differ from today’s reactors primarily by their use of inert gases, molten salt mixtures, or liquid metals to cool the reactor core. Advanced reactors can also consider fuel materials and designs that differ radically from today’s enriched-uranium dioxide pellets within zirconium cladding. Agreement State A U.S. State that has signed an agreement with the U.S. Nuclear Regulatory Commission (NRC) authorizing the State to regulate certain uses of radioactive materials within the State. Atomic energy The energy that is released through a nuclear reaction or radioactive decay process. One kind of nuclear reaction is fission, which occurs in a nuclear reactor and releases energy, usually in the form of heat and radiation. In a nuclear power plant, this heat is used to boil water to produce steam that can be used to drive large turbines. The turbines drive generators to produce electrical power. NUCLEUS FRAGMENT Nuclear Reaction NUCLEUS NEW NEUTRON NEUTRON Background radiation The natural radiation that is always present in the environment. It includes cosmic radiation that comes from the sun and stars, terrestrial radiation that comes from the Earth, and internal radiation that exists in all living things and enters organisms by ingestion or inhalation. The typical average individual exposure in the United States from natural background sources is about 310 millirem (3.1 millisievert) per year. 160 8 GLOSSARY 8 Boiling-water reactor (BWR) A nuclear reactor in which water is boiled using heat released from fission. -

Measurements of Fission Cross Sections for the Isotopes Relevant to the Thorium Fuel Cycle
EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH CERN/INTC 2001-025 INTC/P-145 08 Aug 2001 Measurements of Fission Cross Sections for the Isotopes relevant to the Thorium Fuel Cycle The n_TOF Collaboration ABBONDANNO U.20, ANDRIAMONJE S.10, ANDRZEJEWSKI J.24, ANGELOPOULOS A.12, ASSIMAKOPOULOS P.15, BACRI C-O.8, BADUREK G.1, BAUMANN P.9, BEER H.11, BENLLIURE J.32, BERTHIER B.8, BONDARENKO I.26, BOS A.J.J.36, BUSTREO N.19, CALVINO F.33, CANO-OTT D.28, CAPOTE R.31, CARLSON P.34, CHARPAK G.5, CHAUVIN N.8, CENNINI P.5, CHEPEL V.25, COLONNA N.20, CORTES G.33, CORTINA D.32, CORVI F.23, DAMIANOGLOU D.16, DAVID S.8, DIMOVASILI E.12, DOMINGO C.29, DOROSHENKO A.27, DURAN ESCRIBANO I.32, ELEFTHERIADIS C.16, EMBID M.28, FERRANT L.8, FERRARI A.5, FERREIRA–MARQUES R.25, FRAIS–KOELBL H.3, FURMAN W.26, FURSOV B.27, GARZON J.A.32, GIOMATARIS I.10, GLEDENOV Y.26, GONZALEZ–ROMERO E.28, GOVERDOVSKI A.27, GRAMEGNA F.19, GRIESMAYER E.3, GUNSING F.10, HAIGHT R.37, HEIL M.11, HOLLANDER P.36, IOANNIDES K.G.15, IOANNOU P.12, ISAEV S.27, JERICHA E.1, KADI Y.5, KAEPPELER F.11, KARADIMOS D.17, KARAMANIS D.15, KAYUKOV A.26, KAZAKOV L.27, KELIC A.9, KETLEROV V.27, KITIS G.16, KOEHLER P.E.38., KOPACH Y.26, KOSSIONIDES E.14, KROSHKINA I.27, LACOSTE V.5, LAMBOUDIS C.16, LEEB H.1, LEPRETRE A.10, LOPES M.25, LOZANO M.31, MARRONE S.20, MARTINEZ-VAL J. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Classification of Elements and Periodicity in Properties
74 CHEMISTRY UNIT 3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES The Periodic Table is arguably the most important concept in chemistry, both in principle and in practice. It is the everyday support for students, it suggests new avenues of research to After studying this Unit, you will be professionals, and it provides a succinct organization of the able to whole of chemistry. It is a remarkable demonstration of the fact that the chemical elements are not a random cluster of • appreciate how the concept of entities but instead display trends and lie together in families. grouping elements in accordance to An awareness of the Periodic Table is essential to anyone who their properties led to the wishes to disentangle the world and see how it is built up development of Periodic Table. from the fundamental building blocks of the chemistry, the understand the Periodic Law; • chemical elements. • understand the significance of atomic number and electronic Glenn T. Seaborg configuration as the basis for periodic classification; • name the elements with In this Unit, we will study the historical development of the Z >100 according to IUPAC Periodic Table as it stands today and the Modern Periodic nomenclature; Law. We will also learn how the periodic classification • classify elements into s, p, d, f follows as a logical consequence of the electronic blocks and learn their main configuration of atoms. Finally, we shall examine some of characteristics; the periodic trends in the physical and chemical properties • recognise the periodic trends in of the elements. physical and chemical properties of elements; 3.1 WHY DO WE NEED TO CLASSIFY ELEMENTS ? compare the reactivity of elements • We know by now that the elements are the basic units of all and correlate it with their occurrence in nature; types of matter. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -

Review of Outpatient Brachytherapy Medicare Payments to Carolinas Medical Center
DEPARTMENT OF HEALTH & HUMAN SERVICES OFFICE OF INSPECTOR GENERAL Office of Audit Services, Region IV 61 Forsyth Street, SW, Suite 3T41 Atlanta, GA 30303 February 6, 2012 Report Number: A-04-11-06135 Ms. Suzanne Freeman Divisional President Carolinas Medical Center P.O. Box 32861 Charlotte, NC 28232-2861 Dear Ms. Freeman: Enclosed is the U.S. Department of Health and Human Services (HHS), Office of Inspector General (OIG), final report entitled Review of Outpatient Brachytherapy Medicare Payments to Carolinas Medical Center. We will forward a copy of this report to the HHS action official noted on the following page for review and any action deemed necessary. The HHS action official will make final determination as to actions taken on all matters reported. We request that you respond to this official within 30 days from the date of this letter. Your response should present any comments or additional information that you believe may have a bearing on the final determination. Section 8L of the Inspector General Act, 5 U.S.C. App., requires that OIG post its publicly available reports on the OIG Web site. Accordingly, this report will be posted at http://oig.hhs.gov. If you have any questions or comments about this report, please do not hesitate to call me, or contact Andrew Funtal, Audit Manager, at (404) 562-7762 or through email at [email protected]. Please refer to report number A-04-11-06135 in all correspondence. Sincerely, /Lori S. Pilcher/ Regional Inspector General for Audit Services Enclosure Page 2 – Ms. Suzanne Freeman Direct Reply to HHS Action Official: Nanette Foster Reilly Consortium Administrator Consortium for Financial Management & Fee for Service Operations Centers for Medicare & Medicaid Services 601 East 12th Street, Room 235 Kansas City, Missouri 64106 Department of Health and Human Services OFFICE OF INSPECTOR GENERAL REVIEW OF OUTPATIENT BRACHYTHERAPY MEDICARE PAYMENTS TO CAROLINAS MEDICAL CENTER Daniel R. -

Radiation Quick Reference Guide Recommend Contacting Your State Fusion Center
Domestic Nuclear Detection Office If you encounter something suspicious follow your specific local protocols. Radiation Quick Reference Guide Recommend contacting your state fusion center. DNDO is available 24/7 to assist at 1-877-DNDO-JAC / 1-877-363-6522 JAC Information Line 202-254-7179 Email: [email protected] Nuclear Concerns/ Threats 1. Nuclear Weapon - a device that releases nuclear energy in an ex- Isotopes of Concern for use in RDDs - with common uses plosive manner. Uses Highly Enriched Uranium (HEU) and/or 1. Cobalt-60 – cancer treatment, level/ Plutonium. density gauge, teletherapy, radiography, 2. Improvised Nuclear Device (IND) - a nuclear weapon fabricated food sterilization/irradiation, by a terrorist organization or rogue nation. brachytherapy 2. Iridium-192 – Radiography/non- destructive testing, flaw detection, brachy- therapy “cancer seed”, skin cancer Cobalt 60 sources Uranium “superficial” brachytherapy Plutonium 3. Uranium a. Uranium exists naturally in the earth’s crust. Of the different “isotopes” of uranium, U-235 is the one required to produce a Iridium sentinel and nuclear weapon. gamma camera b. Natural uranium contains a small amount of U-235 (<1%) which Cesium Seeds must be separated in complex extraction processes to create HEU. The predominant uranium isotope is U-238. 3. Cesium-137 - Gauge/level gauge, industrial radiography, brachyther- c. Highly Enriched Uranium (HEU) refers to uranium usable in weap- apy/teletherapy, well logging/density gauges ons due to its enrichment in U-235. 4. Strontium-90 – Radioisotope thermoelectric generator (RTG), fis- d. Approximately 25 kg of HEU is required for a nuclear weapon. sion product, industrial gauges, medical treatment e. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

Uranium Fact Sheet
Fact Sheet Adopted: December 2018 Health Physics Society Specialists in Radiation Safety 1 Uranium What is uranium? Uranium is a naturally occurring metallic element that has been present in the Earth’s crust since formation of the planet. Like many other minerals, uranium was deposited on land by volcanic action, dissolved by rainfall, and in some places, carried into underground formations. In some cases, geochemical conditions resulted in its concentration into “ore bodies.” Uranium is a common element in Earth’s crust (soil, rock) and in seawater and groundwater. Uranium has 92 protons in its nucleus. The isotope2 238U has 146 neutrons, for a total atomic weight of approximately 238, making it the highest atomic weight of any naturally occurring element. It is not the most dense of elements, but its density is almost twice that of lead. Uranium is radioactive and in nature has three primary isotopes with different numbers of neutrons. Natural uranium, 238U, constitutes over 99% of the total mass or weight, with 0.72% 235U, and a very small amount of 234U. An unstable nucleus that emits some form of radiation is defined as radioactive. The emitted radiation is called radioactivity, which in this case is ionizing radiation—meaning it can interact with other atoms to create charged atoms known as ions. Uranium emits alpha particles, which are ejected from the nucleus of the unstable uranium atom. When an atom emits radiation such as alpha or beta particles or photons such as x rays or gamma rays, the material is said to be undergoing radioactive decay (also called radioactive transformation).