An Unusual Disease with an Interesting Sign
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pleural Effusion and Ventilation/Perfusion Scan Interpretation for Acute Pulmonary Embolus
ventricular function by radionuclide angiography during exercise in normal subjects 33. Verani MS. Myocardial perfusion imaging versus two-dimensional echocardiography: and patients with chronic coronary heart disease. J Am Coll Cardiol 1983;1:1518- comparative value in the diagnosis of coronary artery disease. J NucÃCardiol 1529. 1994;1:399-414. 29. Adam WE. Tarkowska A, Bitter F, Stauch M, Geffers H. Equilibrium (gated) 34. Foster T, McNeill AJ, Salustri A, et al. Simultaneous dobutamine stress echocardiog radionuclide ventriculography. Cardiovasc Radial 1979;2:161-173. raphy and technetium-99m SPECT in patients with suspected coronary artery disease. 30. Hurwitz RA, TrêvesS, Kuroc A. Right ventricular and left ventricular ejection fraction J Am Coll Cardiol I993;21:1591-I596. in pediatrie patients with normal hearts: first pass radionuclide angiography. Am 35. Marwick TH, D'Hondt AM, Mairesse GH, Baudhuin T, Wijins W, Detry JM, Meiin Heart J 1984;107:726-732. 31. Freeman ML, Palac R, Mason J, et al. A comparison of dobutamine infusion and JA. Comparative ability of dobutamine and exercise stress in inducing myocardial ischemia in active patients. Br Heart J 1994:72:31-38. supine bicycle exercise for radionuclide cardiac stress testing. Clin NucÃMed 1984:9:251-255. 36. Senior R, Sridhara BS, Anagnostou E, Handler C, Raftery EB, Lahiri A. Synergistic 32. Cohen JL, Greene TO, Ottenweller J, Binebaum SZ, Wilchfort SD, Kim CS. value of simultaneous stress dobutamine sestamibi single-photon-emission computer Dobutamine digital echocardiography for detecting coronary artery disease. Am J ized tomography and echocardiography in the detection of coronary artery disease. -

Pneumothorax Ex Vacuo in a Patient with Malignant Pleural Effusion After Pleurx Catheter Placement
The Medicine Forum Volume 16 Article 20 2015 Pneumothorax ex vacuo in a Patient with Malignant Pleural Effusion After PleurX Catheter Placement Meera Bhardwaj, MS4 Thomas Jefferson University, [email protected] Loheetha Ragupathi, MD Thomas Jefferson University, [email protected] Follow this and additional works at: https://jdc.jefferson.edu/tmf Part of the Medicine and Health Sciences Commons Let us know how access to this document benefits ouy Recommended Citation Bhardwaj, MS4, Meera and Ragupathi, MD, Loheetha (2015) "Pneumothorax ex vacuo in a Patient with Malignant Pleural Effusion After PleurX Catheter Placement," The Medicine Forum: Vol. 16 , Article 20. DOI: https://doi.org/10.29046/TMF.016.1.019 Available at: https://jdc.jefferson.edu/tmf/vol16/iss1/20 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in The Medicine Forum by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Bhardwaj, MS4 and Ragupathi, MD: Pneumothorax ex vacuo in a Patient with Malignant Pleural Effusion After PleurX Catheter Placement Pneumothorax ex vacuo in a Patient with Malignant Pleural Effusion After PleurX Catheter Placement Meera Bhardwaj, MS4 and Loheetha Ragupathi, MD INTRODUCTION Pneumothorax ex vacuo (“without vaccuum”) is a type of pneumothorax that can develop in patients with large pleural effusions. -

An Interesting Case of Undiagnosed Pleural Effusion Case Report
Amit Panjwani, Thuraya Zaid [email protected] Pulmonary Medicine, Salmaniya Medical Complex, Manama, Bahrain. An interesting case of undiagnosed pleural effusion Case report Pleural effusions are commonly encountered in the Investigations revealed a haemoglobin level Cite as: Panjwani A, Zaid T. clinical practise of both respiratory and nonrespiratory of 16.4 g⋅dL−1, and total leukocyte count of An interesting case of specialists. An estimated 1–1.5 million new cases in 8870 cells⋅mm−3 with a differential count of 62% undiagnosed pleural effusion. the USA and 200 000–250 000 new cases of pleural neutrophils, 28% lymphocytes, 7% monocytes, 2% Breathe 2017; 13: e46–e52. effusions are reported from the UK each year [1]. eosinophils and 1% basophils. The platelet count Analysis of the relevant clinical history, physical was 160 000 cells⋅mm−3. Creatinine, electrolytes examination, chest radiography and diagnostic and liver function tests were normal. The ECG was thoracentesis is useful in identifying the cause of unremarkable and cardiac enzymes were within pleural effusion in majority of the cases [2]. In a few normal limits. Chest radiograph (figure 1) showed a cases, the aetiology may be unclear after the initial mild, right-sided pleural effusion, blunting of the left assessment. The list of diseases that may account for costophrenic angle, no shift of mediastinal position a persistent undiagnosed pleural effusion is long [3]. and no lung parenchymal opacities. We present an interesting case of undiagnosed pleural effusion that was encountered in our hospital. R Case presentation A 33-year-old male presented to our hospital with a history of sudden-onset, pleuritic, right-sided chest pain of 2 days’ duration. -

COVID-19 Pneumonia: the Great Radiological Mimicker
Duzgun et al. Insights Imaging (2020) 11:118 https://doi.org/10.1186/s13244-020-00933-z Insights into Imaging EDUCATIONAL REVIEW Open Access COVID-19 pneumonia: the great radiological mimicker Selin Ardali Duzgun* , Gamze Durhan, Figen Basaran Demirkazik, Meltem Gulsun Akpinar and Orhan Macit Ariyurek Abstract Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide since December 2019. Although the reference diagnostic test is a real-time reverse transcription-polymerase chain reaction (RT-PCR), chest-computed tomography (CT) has been frequently used in diagnosis because of the low sensitivity rates of RT-PCR. CT fndings of COVID-19 are well described in the literature and include predominantly peripheral, bilateral ground-glass opacities (GGOs), combination of GGOs with consolida- tions, and/or septal thickening creating a “crazy-paving” pattern. Longitudinal changes of typical CT fndings and less reported fndings (air bronchograms, CT halo sign, and reverse halo sign) may mimic a wide range of lung patholo- gies radiologically. Moreover, accompanying and underlying lung abnormalities may interfere with the CT fndings of COVID-19 pneumonia. The diseases that COVID-19 pneumonia may mimic can be broadly classifed as infectious or non-infectious diseases (pulmonary edema, hemorrhage, neoplasms, organizing pneumonia, pulmonary alveolar proteinosis, sarcoidosis, pulmonary infarction, interstitial lung diseases, and aspiration pneumonia). We summarize the imaging fndings of COVID-19 and the aforementioned lung pathologies that COVID-19 pneumonia may mimic. We also discuss the features that may aid in the diferential diagnosis, as the disease continues to spread and will be one of our main diferential diagnoses some time more. -

Vocal Cord Dysfunction: Analysis of 27 Cases and Updated Review of Pathophysiology & Management
THIEME Original Research 125 Vocal Cord Dysfunction: Analysis of 27 Cases and Updated Review of Pathophysiology & Management Shibu George1 Sandeep Suresh2 1 Department of ENT, Government Medical College, Kottayam, Kerala, India Address for correspondence ShibuGeorge,MS,DNB,Charivukalayil 2 Department of ENT, Little Flower Hospital, Ernakulam, Kerala, India (House), Ettumanoor, Kottayam 686631, Kerala, India (e-mail: [email protected]; [email protected]). Int Arch Otorhinolaryngol 2019;23:125–130. Abstract Introduction Vocal cord dysfunction is characterized by unintentional paradoxical vocal cord movement resulting in abnormal inappropriate adduction, especially during inspiration; this predominantly manifests as unresponsive asthma or unexplained stridor. It is prudent to be well informed about the condition, since the primary presentation may mask other airway disorders. Objective This descriptive study was intended to analyze presentations of vocal cord dysfunction in a tertiary care referral hospital. The current understanding regarding the pathophysiology and management of the condition were also explored. Methods A total of 27 patients diagnosed with vocal cord dysfunction were analyzed based on demographic characteristics, presentations, associations and examination findings. The mechanism of causation, etiological factors implicated, diagnostic considerations and treatment options were evaluated by analysis of the current literature. Results Therewasastrongfemalepredilection noted among the study population (n ¼ 27), which had a mean age of 31. The most common presentations were stridor Keywords (44%) and refractory asthma (41%). Laryngopharyngeal reflux disease was the most ► Vocal Cord common association in the majority (66%) of the patients, with a strong overlay of Dysfunction anxiety, demonstrable in 48% of the patients. ► paradoxical vocal Conclusion Being aware of the condition is key to avoid misdiagnosis in vocal cord cord motion dysfunction. -

Middle Airway Obstructiondit May Be Happening Under Our Noses Philip G Bardin,1 Sebastian L Johnston,2 Garun Hamilton1
Thorax Online First, published on July 19, 2012 as 10.1136/thoraxjnl-2012-202221 Chest clinic OPINION Thorax: first published as 10.1136/thoraxjnl-2012-202221 on 19 July 2012. Downloaded from Middle airway obstructiondit may be happening under our noses Philip G Bardin,1 Sebastian L Johnston,2 Garun Hamilton1 < Additional materials are ABSTRACT for this conceptual error to be refuted.2 The virus is published online only. To view Background Lower airway obstruction has evolved to now understood to spread from nose to lung, and these files please visit the denote pathologies associated with diseases of the lung, appropriate recognition of the close association journal online (http://dx.doi.org/ 10.1136/ whereas, conditions proximal to the lung embody upper between upper and lower airway pathologies has thoraxjnl-2012-202221/content/ airway obstruction. This approach has disconnected had positive outcomes. An example is novel strat- early/recent). diseases of the larynx and trachea from the lung, and egies that may not be able to prevent colds, but 1Lung and Sleep Medicine, removed the ‘middle airway’ from the interest and that could ameliorate virus asthma exacerbations Monash University and Hospital involvement of respiratory physicians and scientists. through the use of inhaled interferon.3 and Monash Institute of Medical However, recent studies have indicated that dysfunction Accumulating evidence suggests that the middle Research (MIMR), Melbourne, of this anatomical region may be a key component of Australia airway plays a key role in airway obstruction either 2Respiratory Medicine, Imperial overall airway obstruction, either independently or in independently or with coexisting lung disease. -
Mechanical Ventilation Guide
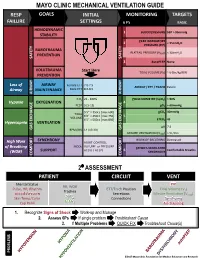
MAYO CLINIC MECHANICAL VENTILATION GUIDE RESP GOALS INITIAL MONITORING TARGETS FAILURE SETTINGS 6 P’s BASIC HEMODYNAMIC 1 BLOOD PRESSURE SBP > 90mmHg STABILITY PEAK INSPIRATORY 2 < 35cmH O PRESSURE (PIP) 2 BAROTRAUMA PLATEAU PRESSURE (P ) < 30cmH O PREVENTION PLAT 2 SAFETY SAFETY 3 AutoPEEP None VOLUTRAUMA Start Here TIDAL VOLUME (V ) ~ 6-8cc/kg IBW PREVENTION T Loss of AIRWAY Female ETT 7.0-7.5 AIRWAY / ETT / TRACH Patent Airway MAINTENANCE Male ETT 8.0-8.5 AIRWAY AIRWAY FiO2 21 - 100% PULSE OXIMETRY (SpO2) > 90% Hypoxia OXYGENATION 4 PEEP 5 [5-15] pO2 > 60mmHg 5’5” = 350cc [max 600] pCO2 40mmHg TIDAL 6’0” = 450cc [max 750] 5 VOLUME 6’5” = 500cc [max 850] ETCO2 45 Hypercapnia VENTILATION pH 7.4 GAS GAS EXCHANGE BPM (RR) 14 [10-30] GAS EXCHANGE MINUTE VENTILATION (VMIN) > 5L/min SYNCHRONY WORK OF BREATHING Decreased High Work ASSIST CONTROL MODE VOLUME or PRESSURE of Breathing PATIENT-VENTILATOR AC (V) / AC (P) 6 Comfortable Breaths (WOB) SUPPORT SYNCHRONY COMFORT COMFORT 2⁰ ASSESSMENT PATIENT CIRCUIT VENT Mental Status PIP RR, WOB Pulse, HR, Rhythm ETT/Trach Position Tidal Volume (V ) Trachea T Blood Pressure Secretions Minute Ventilation (V ) SpO MIN Skin Temp/Color 2 Connections Synchrony ETCO Cap Refill 2 Air-Trapping 1. Recognize Signs of Shock Work-up and Manage 2. Assess 6Ps If single problem Troubleshoot Cause 3. If Multiple Problems QUICK FIX Troubleshoot Cause(s) PROBLEMS ©2017 Mayo Clinic Foundation for Medical Education and Research CAUSES QUICK FIX MANAGEMENT Bleeding Hemostasis, Transfuse, Treat cause, Temperature control HYPOVOLEMIA Dehydration Fluid Resuscitation (End points = hypoxia, ↑StO2, ↓PVI) 3rd Spacing Treat cause, Beware of hypoxia (3rd spacing in lungs) Pneumothorax Needle D, Chest tube Abdominal Compartment Syndrome FLUID Treat Cause, Paralyze, Surgery (Open Abdomen) OBSTRUCTED BLOOD RETURN Air-Trapping (AutoPEEP) (if not hypoxic) Pop off vent & SEE SEPARATE CHART PEEP Reduce PEEP Cardiac Tamponade Pericardiocentesis, Drain. -

Allergic Bronchopulmonary Aspergillosis: a Perplexing Clinical Entity Ashok Shah,1* Chandramani Panjabi2
Review Allergy Asthma Immunol Res. 2016 July;8(4):282-297. http://dx.doi.org/10.4168/aair.2016.8.4.282 pISSN 2092-7355 • eISSN 2092-7363 Allergic Bronchopulmonary Aspergillosis: A Perplexing Clinical Entity Ashok Shah,1* Chandramani Panjabi2 1Department of Pulmonary Medicine, Vallabhbhai Patel Chest Institute, University of Delhi, Delhi, India 2Department of Respiratory Medicine, Mata Chanan Devi Hospital, New Delhi, India This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. In susceptible individuals, inhalation of Aspergillus spores can affect the respiratory tract in many ways. These spores get trapped in the viscid spu- tum of asthmatic subjects which triggers a cascade of inflammatory reactions that can result in Aspergillus-induced asthma, allergic bronchopulmo- nary aspergillosis (ABPA), and allergic Aspergillus sinusitis (AAS). An immunologically mediated disease, ABPA, occurs predominantly in patients with asthma and cystic fibrosis (CF). A set of criteria, which is still evolving, is required for diagnosis. Imaging plays a compelling role in the diagno- sis and monitoring of the disease. Demonstration of central bronchiectasis with normal tapering bronchi is still considered pathognomonic in pa- tients without CF. Elevated serum IgE levels and Aspergillus-specific IgE and/or IgG are also vital for the diagnosis. Mucoid impaction occurring in the paranasal sinuses results in AAS, which also requires a set of diagnostic criteria. Demonstration of fungal elements in sinus material is the hall- mark of AAS. -

CHEST RADIOLOGY: Goals and Objectives
Harlem Hospital Center Department of Radiology Residency Training Program CHEST RADIOLOGY: Goals and Objectives ROTATION 1 (Radiology Years 1): Resident responsibilities: • ED chest CTs • Inpatient and outpatient plain films including the portable intensive care unit radiographs • Consultations with referring clinicians MEDICAL KNOWLEDGE: • Residents must demonstrate knowledge about established and evolving biomedical, clinical, and cognitive sciences and the application of this knowledge to patient care. At the end of the rotation, the resident should be able to: • Identify normal radiographic and CT anatomy of the chest • Identify and describe common variants of normal, including aging changes. • Demonstrate a basic knowledge of radiographic interpretation of atelectasis, pulmonary infection, congestive heart failure, pleural effusion and common neoplastic diseases of the chest • Identify the common radiologic manifestation of thoracic trauma, including widened mediastinum, signs of aortic laceration, pulmonary contusion/laceration, esophageal and diaphragmatic rupture. • Know the expected postoperative appearance in patients s/p thoracic surgery and the expected location of the life support and monitoring devices on chest radiographs of critically ill patients (intensive care radiology); be able to recognize malpositioned devices. • Identify cardiac enlargement and know the radiographic appearance of the dilated right vs. left atria and right vs. left ventricles, and pulmonary vascular congestion • Recognize common life-threatening -

Pathology of Allergic Bronchopulmonary Aspergillosis
[Frontiers in Bioscience 8, e110-114, January 1, 2003] PATHOLOGY OF ALLERGIC BRONCHOPULMONARY ASPERGILLOSIS Anne Chetty Department of Pediatrics, Floating Hospital for Children, New England Medical Center, Boston, MA TABLE OF CONTENTS 1. Abstract 2. Introduction 3. Immunopathogenesis 4. Pathologic Findings 4.1. Plastic bronchitis 4.2. Allergic fungal sinusitis 4.3. ABPA in cystic fibrosis 5. Acknowledgement 6. References 1. ABSTRACT Allergic bronchopulmonary aspergillosis (ABPA) individuals with episodic obstructive lung diseases such as occurs in patients with asthma and cystic fibrosis when asthma and cystic fibrosis that produce thick, tenacious Aspergillus fumigatus spores are inhaled and grow in sputum. bronchial mucus as hyphae. Chronic colonization of Aspergillus fumigatus and host’s genetically determined Decomposing organic matter serves as a substrate immunological response lead to ABPA. In most cases, for the growth of Aspergillus species. Because biologic lung biopsy is not necessary because the diagnosis is made heating produces temperatures as high as 65° to 70° C, on clinical, serologic, and roentgenographic findings. Some Aspergillus spores will not be recovered in the latter stages patients who have had lung biopsies or partial resections of composting. Aspergillus species have been recovered for atelectasis or infiltrates will have histologic diagnoses. from potting soil, mulches, decaying vegetation, and A number of different histologic diagnoses can be found sewage treatment facilities, as well as in outdoor air and even in the same patient. In the early stages the bronchial Aspergillus spores grow in excreta from birds (1) wall is infiltrated with mononuclear cells and eosinophils. Mucoid impaction and eosinophilic pneumonia are seen Allergic fungal pulmonary disease is manifested subsequently. -

The Spectrum of Computed Tomography Appearances
RESPIRATORY MEDICIXE (1997) 91, 213-219 Allergic bronchopulmonary aspergillosis: the spectrum of computed tomography appearances N. PANCHAL",R. BHAGAT",C. PANT+AND A. SHAH" “Department of Clinical Research, Vallabhbhai Pate1 Chest Institute, University of Delhi, Delhi, lndia ‘Imaging Division, Institute of Nuclear Medicine and Allied Sciences, Timaupuv, Delhi, India Although computed tomography (CT) of the thorax has been compared to plain chest radiography and bronchography for demonstration of central bronchiectasis (CB) in allergic bronchopulmonary aspergil- losis (ABPA), the CT presentation of the disease is yet to be highlighted. With this in view, the CT appearances in 23 patients with ABPA were evaluated. The scans were assessed for bronchial, parenchymal and pleural abnormalities. Central bronchiectasis was identified in all patients, involving 114 (85%) of the 134 lobes and 210 (52%) of the 406 segments studied. Other bronchial abnormalities such as dilated and totally occluded bronchi (11 patients), air-fluid levels within dilated bronchi (five patients), bronchial wall thickening (10 patients) and parallel-line shadows (seven patients) were also observed. Parenchymal abnormalities, which had a predilection for upper lobes, included consolidation in 10 (43%) patients, collapse in four (17%) patients and parenchymal scarring in 19 (83%) patients. A total of six cavities were seen in three (13%) patients, and an emphysematous bullae was detected in one (4%) patient. The pleura was involved in 10 (43%) patients. Ipsilateral pleural effusion with collapse was observed in one patient, while in nine other patients, parenchymal lesions extended up to the pleura. Concomitant allergic Aspergillus sinusitis (AAS) was also detected in three (13%) of the 23 patients. -

Percutaneous Endoscopic Gastrostomy Versus Nasogastric Tube Feeding: Oropharyngeal Dysphagia Increases Risk for Pneumonia Requiring Hospital Admission
nutrients Article Percutaneous Endoscopic Gastrostomy versus Nasogastric Tube Feeding: Oropharyngeal Dysphagia Increases Risk for Pneumonia Requiring Hospital Admission Wei-Kuo Chang 1,*, Hsin-Hung Huang 1, Hsuan-Hwai Lin 1 and Chen-Liang Tsai 2 1 Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan; [email protected] (H.-H.H.); [email protected] (H.-H.L.) 2 Division of Pulmonary and Critical Care, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-2-23657137; Fax: +886-2-87927138 Received: 3 November 2019; Accepted: 4 December 2019; Published: 5 December 2019 Abstract: Background: Aspiration pneumonia is the most common cause of death in patients with percutaneous endoscopic gastrostomy (PEG) and nasogastric tube (NGT) feeding. This study aimed to compare PEG versus NGT feeding regarding the risk of pneumonia, according to the severity of pooling secretions in the pharyngolaryngeal region. Methods: Patients were stratified by endoscopic observation of the pooling secretions in the pharyngolaryngeal region: control group (<25% pooling secretions filling the pyriform sinus), pharyngeal group (25–100% pooling secretions filling the pyriform sinus), and laryngeal group (pooling secretions entering the laryngeal vestibule). Demographic data, swallowing level scale score, and pneumonia requiring hospital admission were recorded. Results: Patients with NGT (n = 97) had a significantly higher incidence of pneumonia (episodes/person-years) than those patients with PEG (n = 130) in the pharyngeal group (3.6 1.0 ± vs. 2.3 2.1, P < 0.001) and the laryngeal group (3.8 0.5 vs.