Pacific Chrysophylloideae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Pouteria Sapota, Sapotaceae) from Southeastern Mexico: Its Putative Domestication Center
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 7-6-2019 Structure and genetic diversity in wild and cultivated populations of Zapote mamey (Pouteria sapota, Sapotaceae) from southeastern Mexico: its putative domestication center Jaime Martínez-Castillo Centro de Investigación Científica de ucatánY (CICY), [email protected] Nassib H. Blancarte-Jasso Centro de Investigación Científica de ucatánY (CICY) Gabriel Chepe-Cruz Centro de Investigación Científica de ucatánY (CICY) Noemí G. Nah-Chan Centro de Investigación Científica de ucatánY (CICY) Matilde M. Ortiz-García Centro de Investigación Científica de ucatánY (CICY) See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Martínez-Castillo, Jaime; Blancarte-Jasso, Nassib H.; Chepe-Cruz, Gabriel; Nah-Chan, Noemí G.; Ortiz- García, Matilde M.; and Arias, Renee S., "Structure and genetic diversity in wild and cultivated populations of Zapote mamey (Pouteria sapota, Sapotaceae) from southeastern Mexico: its putative domestication center" (2019). Publications from USDA-ARS / UNL Faculty. 2200. https://digitalcommons.unl.edu/usdaarsfacpub/2200 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Jaime Martínez-Castillo, Nassib H. -

Pouteria Sapota
Pouteria sapota Pouteria sapota, mamey sapote, is a species of tree na- propagated by grafting, which ensures the new plant has tive to Central America, naturally ranging from southern the same characteristics as the parent, especially its fruit. Mexico to southern Costa Rica. Today, the tree is cul- It is also considerably faster than growing trees by seed. tivated not only in Mexico, but also in Central America, The leaves are pointed at both ends, 4 to 12 inches in the Caribbean, and South Florida for its fruit, which is length and grow in clusters at the ends of branches. commonly eaten in many Latin American countries. It has different names depending on the country: mamey The fruit is about 10 to 25 cm (4 to 10 inches) long and (Cuba), zapote colorado (Costa Rica), níspero and zapote 8 to 12 cm (3 to 5 inches) wide and has flesh ranging in rojo (South America), among others. color from pink to orange to red. The brown skin has a texture somewhat between sandpaper and the fuzz on a peach. The fruit’s texture is creamy and soft. A mamey 1 Description sapote is ripe when the flesh is pink when a fleck of the skin is removed. The flesh should give slightly, as with a ripe kiwifruit. The mamey sapote is related to other sapotes such as sapodilla (Manilkara zapota), abiu (P. caimito) and canistel (P. campechiana), but unrelated to the black sapote (Diospyros digyna) and white sapote (Casimiroa edulis).[2] It should not be confused with the mammee ap- ple (Mammea americana). -

Renata Gabriela Vila Nova De Lima Filogenia E Distribuição
RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) RECIFE 2019 RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) Dissertação apresentada ao Programa de Pós-graduação em Botânica da Universidade Federal Rural de Pernambuco (UFRPE), como requisito para a obtenção do título de Mestre em Botânica. Orientadora: Carmen Silvia Zickel Coorientador: André Olmos Simões Coorientadora: Liliane Ferreira Lima RECIFE 2019 Dados Internacionais de Catalogação na Publicação (CIP) Sistema Integrado de Bibliotecas da UFRPE Biblioteca Central, Recife-PE, Brasil L732f Lima, Renata Gabriela Vila Nova de Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae) / Renata Gabriela Vila Nova de Lima. – 2019. 98 f. : il. Orientadora: Carmen Silvia Zickel. Coorientadores: André Olmos Simões e Liliane Ferreira Lima. Dissertação (Mestrado) – Universidade Federal Rural de Pernambuco, Programa de Pós-Graduação em Botânica, Recife, BR-PE, 2019. Inclui referências e anexo(s). 1. Mata Atlântica 2. Filogenia 3. Plantas florestais 4. Sapotaceae I. Zickel, Carmen Silvia, orient. II. Simões, André Olmos, coorient. III. Lima, Liliane Ferreira, coorient. IV. Título CDD 581 ii RENATA GABRIELA VILA NOVA DE LIMA Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae Juss.) Dissertação apresentada e -

Amazon Alive!
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Tbiseries3.Pdf
b_[^LZE[aâ aL_QLaâ 5â bxâb¶¬²x§o¬Àâ ax¶xÀâ ²¶xÀx§ÅÀâÅxâ¶xÀÊÅÀâ¬|âÀÅÊuxÀâ j§uâ ¶xÀxj¶qâ jqÅÎÅxÀâ¶xjÅxuâŬâÅxâ q¬§Àx¶Îjà Ŭ§âj§uâÑÀxâÊÃÛjŬ§â¬|â¶xÀÅâj§uÀâ § âÅxâ Ê¢uâŶ¬²qÀ#âbxâ Àx¶xÀâq¬§Å§ÊxÀâj§uâ§Åx¶jÅxÀâÅxâ }®¶¢x¶âb¶¬²x§p¬Àâaqx§Åqâj§uâbxq§qjâax¶xÀ#âbxâÀÅÊuxÀâ²ÊoÀxuâ§âÅÀâÀx¶xÀâjÎxâoxx§âqj¶à ¶xuâ ¬ÊÅâÑŧâ Åxâ §Åx¶§jŬ§j âb¶¬²x§o¬ÀⲶ¬¶j¢¢x#â[qqjÀ¬§jÕ âÅÀâÀx¶xÀâ¢jÕⲶxÀx§ÅâÅxâ ¶xÀÊÅÀâ¬|â¬Åx¶âÀÅÊuxÀâÒqâq¬§Å¶oÊÅxâŬâÅxâ¬oxqÅÎxÀâ¬|âÅxâb¶¬²x§o¬ÀⲶ¬¶j¢¢x+â GQ^KDbDâ T\YQZTVQRULâ EQEVQ[bPLLTâKLYâPDDNâ aÅxxxâPj§ÀâÅz¶â ^jÅÅx¶§Àâ §â Ŷ¬´qjâ ¶j§â}®¶xÀÅâ§â NÊÕj§jâ5ĺPj§Àâ Åx¶âaÅxxx#â fjx§§x§?âbxâb¶¬²x§o¬ÀâM¬Ê§ujÞ Å¬§#â Qâčĺ1â  c¶¬²x§o¬ÀâÀx¶xÀâ bxÀÀâ_ÀʧÎx¶ÀÅxÅâdŶxqÅ#â fÅâ¶x ,â fÅâÀÊ¢¢j¶Õâ§â KÊÅq$â QaEZâ=.8125.188â aÊoxqÅâxju§À@âŶ¬²qj â¶j§â}®¶xÀÅÀBâ NÊÕj§jâ5ĺ ²x§¬¬Õ#â Ģĺ 1==5â aÅqçâb¶¬²x§o¬Àâ D â¶ÅÀâ¶xÀx¶Ïxu#âY¬â²j¶Åâ¬|âÅÀâ²ÊoqjŬ§ âj²j¶Å⬢âoo¬¶j²qâujÅjâo ¶x|âµÊ¬ÅjŬ§Àâ§â q¶Åqjâ¶xÎxÒÀâ ¢jÕâoxâ¶x²¶¬uÊqxuâ ¶x¶xq¬¶uxu⬶â²ÊoÀxuâ § âj§Õâ}®¶¢â §qÊu§â²¶§ÅⲬŬà q¬²Õ â¢q¶¬}®¶¦ âxxqŶ¬§q⬶âxxqŶ¬¢j§xÅqâ¶xq¬¶uâÒŬÊÅâÒ¶ÅÅx§â²x¶¢ÀÀ¬§â G¬Îx¶âuxÀ§Aâ Kj¢¬§uâG¬¢¢Ê§qjŬ§â ^¶§ÅxuâoÕ?â exx§¢j§âK¶Êx¶À âihjx§§x§#â G¬Îx¶â²¬Å¬âªÀxÅ@â fjjojâ¶xÀÅⲬŬâoÕâPj§ÀâÅx¶âaÅxxx+â $DD,@6BM26MD@9>2($3M @$26M-9@,BDM26MEH$6$M JƗƗ°L ƗƗƗ/×Ɨ $ƗƗƗƗ °ƗRƗ %Ɨ ĵHĴ_5èįæHIJ5ĹƗ Ɨ0ƗƗ Ɨ ƗƗ LƗ Ɨ ƗH0Ɨ°ƗpL Ɨ ƗWƗƗ ƗHLƗ6íŪLàƗJ*BGG8GƗƗ/0 àƗ Ɨ °Ɨ ƗƗ Ɨ< ƗƗB0Ɨ Ɨ ƗƗƗ ŊƗƗ Ɨ1AƗƗƗ1PƗ ƗƗƗ19;P9Ɨ Ɨ æŶƗýºžƗèýººŢºƗ ƗƗ@&ƗƗ1µƗƗ'L Ɨ ]¶¬¢¬Å{¶>â ]¶¬|#J¶#X S)D"â fy¶y¶â Îy¶n¬¨uy¨âjj¨âvyâMjqÊÅyÅâE¬¬yâÎj¨âwyâ d¨Îy¶ÁÅyÅâdŶyqÅâ byâ¨ÎyÁÅjŬ¨Áâ¶y²¬¶Åyuâ¨â ÅÁâÅyÁÁâÐy¶yâqj¶¶yuâ ¬ÊÅâjÅâÅyâb¶¬²y¨n¬Áâ]¶¬¶j¢¢yâN ÊÖj¨j â 14LâNj¶¨yÅÅÁŶyzÅ -

Wood Anatomy of Neotropical Sapotaceae VI Chloroluma
WOOD ANATOMY OF THE NEOTROPICAL SAPOTACEAE VlI. CHRYSOPHYLLUM RESEARCH PAPER FPL 331 FOREST PRODUCTS LABORATORY FOREST SERVICE U.S. DEPARTMENT OF AGRICULTURE MADISON, WIS. 1978 Preface The Sapotaceae form an important part of the ecosystem in the neotropics; forexample, limited inventories made in the Amazon Basin indicate that this family makes up about 25% of the standing timber volume there. This would represent an astronomical volume of timber but at present only a very small fraction is being utilized. Obviously, better information would help utilization--expecially if that information can result in clear identification of species. The Sapotaceae represent a well-marked and natural family but the homogeneous nature of their floral characters makes generic identifi cation extremely difficult. This in turn is responsible for the extensivesynonomy. Baehni and Bernardi state the situation with respect to Peru but this would hold equally well for all of the neotropics: "For instance, of the 39 species and one variety described hereunder, 13 are known only from the Peruvian type; and 23 taxa here presented have no fruit or seed. It is universally admitted that the taxonomy of this family is almost impossible without--for the same species--leaves, flowers, fruits, and seeds." Unfortunately, species continue to be named on the basis of flowering or fruiting material alone and this continues to add to the already confused state of affairs. This paper on Chrysophyllum is the seventh in a series describing the anatomy of the secondary xylem of the neotropical Sapotaceae. The earlier papers, all by the same author and under the same general heading,include: I. -

The Identity of Ucuqui by Joao Murca Fires1 and Richard Evans Schultes2
THE IDENTITY OF UCUQUI BY JOAO MURCA FIRES1 AND RICHARD EVANS SCHULTES2 ONE of the results of recent field work in the upper Rio Negro basin of Brazil has been the identification of a useful plant of that area—the ucuqui. The fruit of this tree has an edible and delicious mesocarp and is an impor- tant part of the diet of the native peoples of the region. Investigation has shown that the ucuqui is an unde- scribed species of the sapotaceous genus Pouteria. It is altogether fitting that, in publishing a description of this food plant, we employ as a specific epithet the common name which refers exclusively to this species over the greater part of its range. Pouteria Ucuqui is immediately set apart from all other species of the genus by the excessively developed disk which surrounds the ovary. Pouteria Ucuqui Pi res &, Schultes sp. nov. Arbor enormis, usque ad centum viginti pedes alta, radicibus tabularibus, trunco columnari usque ad tres pedes in diametro, cortice crasso, molli, extus atrobadio 1 Chief, Section of Biology, Instituto Agronomico do Norte, Belem do Para, Brazil. * Botanist, Bureau of Plant Industry, Soils, and Agricultural Engi- neering, Agricultural Research Administration, U.S. Department of Agriculture; Research Fellow, Botanical Museum, Harvard Univer- sity ; Collaborator, Instituto Agronomico do Norte. [87] et intus sanguineo cum latice alho aquosoque. Hamuli juniores, inflorescentiae, petioli et foliorum nervi indu- mento ferrugineo-pulverulento vel ferrugineo-furfuraceo obtecti. Folia alterna, bene coriacea, elliptica, basi et apice acuta vel obtusiuscula, plerumque cum acumine 7-10 mm. longo, margine Integra, 11—20 cm. -
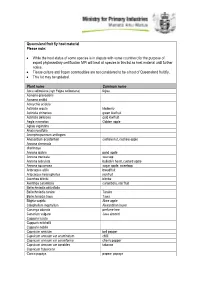
MPI Queensland Fruit Fly Host Material List
Queensland fruit fly host material Please note: While the host status of some species is in dispute with some countries; for the purpose of export phytosanitary certification MPI will treat all species in this list as host material until further notice. Tissue culture and frozen commodities are not considered to be a host of Queensland fruit fly. This list may be updated. Plant name Common name Acca sellowiana (syn Feijoa sellowiana) feijoa Acmena graveolens Acmena smithii Acroychia acidula Actinidia arguta kiwiberry Actinidia chinensis green kiwifruit Actinidia deliciosa gold kiwifruit Aegle marmelos Golden apple Aglaia sapindina Alyxia ruscifolia Amorphospermum antilogum Anacardium occidentale cashew nut, cashew apple Annona cherimola cherimoya Annona glabra pond apple Annona muricata soursop Annona reticulata bullock's heart, custard apple Annona squamosa sugar apple, sweetsop Artocarpus altilis breadfruit Artocarpus heterophyllus jackfruit Averrhoa bilimbi blimbe Averrhoa carambola carambola, star fruit Beilschmiedia obtusifolia Beilschmiedia taraire Taraire Beilschmiedia tawa Tawa Blighia sapida Akee apple Calophyllum inophyllum Alexandrian laurel Cananga odorata perfume tree Canarium vulgare Java almond Capparis lucida Capparis mitchellii Capparis nobilis Capsicum annuum bell pepper Capsicum annuum var acuminatum chilli Capsicum annuum var cerasiforme cherry pepper Capsicum annuum var conoides tabasco Capsicum frutescens Carica papaya papaw, papaya Carica pentagona babaco Carissa ovata Casimiroa edulis white sapote Cassine australia Castanospora alphandii Chrysophyllum cainito caimito, star apple Cissus sp Citrullus lanatus Watermelon Citrus spp. Citrus aurantiifolia West indian lime Citrus aurantium Seville orange Citrus grandis (syn maxima) pummelo Citrus hystrix kaffir lime Citrus jambhiri rough lemon Citrus latifolia Tahatian lime Citrus limetta sweet lemon tree Citrus limon lemon Citrus maxima (syn grandis) pomelo, shaddock Citrus medica citron Citrus meyeri Meyer lemon Citrus reticulata mandarin Citrus reticulata var. -

Price List: Plant Species March 2016
PRICE LIST: PLANT SPECIES MARCH 2016 NOOSA & DISTRICT LANDCARE RESOURCE CENTRE ABN: 73 315 096 794 Station Street, POMONA (opposite the pub) Ph: (07) 5485 2468 Open: Wednesday – Friday 09:30am to 2:30pm Saturday 09:00am to 12:00pm (midday) TUBE STOCK PRICES (including GST) Tube stock: $2.00 Super tubes: $3.00 * Orders 100-500: $1.80 Kauri, Brown & Hoop pines: $2.20 * Orders 500 plus: $1.54 Bunya pines: $3.50 * Orders over 1000 – price negotiable Specials: $1.00 * Larger pots as marked ** Members receive 10% - 20% discount on plants** Please phone 5485 2468 for enquiries or come in and see us today! Plant Species Acacia bakeri MARBLEWOOD NEW Quick growing medium rainforest tree to 10 metres with stunning red-tipped new growth and bushy foliage. Fluffy pale yellow flower heads in spring. Acacia flavescens PRIMROSE BALL WATTLE Shrub to 4m throughout Queensland and Nsw, mostly in Coastal areas. Oftent very ‘showy’ flowering periods in June with cream coloured ball-flowers. Unusal seed pods show the seeds very obviously which attracts birds such as the pale headed rosella. Page 1 of 12 Acacia fimbriata BRISBANE WATTLE Shrub or bushy small tree to 4m. Hardy and fast growing. Attractive ferny semi-weeping foliage. Flowers are scented yellow fluffy balls in winter. Acacia melanoxylon BLACKWOODN NEW Hardy, fast growing medium sized tree to 20 metres and long lived. Pale cream pom-pom flowers in the warmer months Grows rapidily into a thickly crowned tree and prefers a sunny position, pruning or thinning may be required. The golden brown timber is one of Australia’s best for cabinet work and ornamental interiors. -

Following D U B a R D, the Considered Shape of the Embryo Is a Good Taxonomic Character
Notes on Guiana Sapotaceae by P. J. Eyma (Utrecht). the work Notwithstanding large amount of spent by several botanists this does not satis- on family, taxonomy appear very and has factory, a general agreement on generic limits not yet been reached. The result has been a perplexing number of generic and sectional The author for his names. present apologizes adding the number of to interpretations. This study of American Sapotaceae, primarily undertaken in connection with the Flora of Surinam, could not have been com- without the loan of the pleted generous specimens by herbaria at Brussels [B], Berlin —Dahlem [D], Kew [K], and Leyden [L]. the author short visit to the herbaria In 1934 paid a at Brussels [B] The collections and at Paris [P]. of this family at Paris are of special interest owing to the fact that they contain the material B i 11 Pierre and and studied by a on, Du'bard, bear numer- and ous notes analytical drawings, especially by Pierre, attached British to the sheets. A number of Guiana Sapotaceae from the Kew Herbarium was received for determination shortly afterwards. The author feels greatly indebted to the directors of the above their kind and mentioned Herbaria for hel{), particularly to Prof. Dr. P 11 Utrecht, under whose direction this A. u e, study was undertaken. Unless otherwise mentioned the specimens cited are in the Utrecht Herbarium [U]. The principial alterations in the classification of Sapotaceae in this due the for the paper are to rejection classifying purposes of certain number of flower-parts and, to a degree, of the staminodial development also. -

Short Communication
Biota Neotropica 20(1): e20190815, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Short Communication A new synonym for Micropholis gardneriana (Sapotaceae) with complete description, anatomy and distribution notes Angélica Cândida Ferreira1* , Josiane Silva Araújo2 , Eduardo Bezerra de Almeida Jr3 & Carmen Silvia Zickel1 1Universidade Federal Rural de Pernambuco, Departamento de Biologia, Pós-graduação em Botânica, 52171-900, Recife, PE, Brasil 2Universidade Estadual do Piauí, Departamento de Biologia, 64280-000, Campo Maior, PI, Brasil 3Universidade Federal do Maranhão, Departamento de Biologia, 65080-805, São Luís, MA, Brasil *Corresponding author: Angélica Cândida Ferreira, e-mail: [email protected] Ferreira, A.C.; Araújo, J.S.; Almeida Jr., E.B.; Zickel, C.S. A new synonym for Micropholis gardneriana (Sapotaceae) with complete description, anatomy and distribution notes. Biota Neotropica. 20(1): e20190815. http://dx.doi.org/10.1590/1676-0611-BN-2019-0815 Abstract: In the present work, we synonymize Micropholis compta under M. gardneriana due to the overlap of morphoanatomical characters and the absence of distinctive attributes, verified during taxonomic and anatomical study of the genus Micropholis for Brazil. This study provides an updated description of M. gardneriana, including macro- and micro-morphological data, a distribution map, and comments on conservation status, ecological and taxonomy. Keywords: Chrysophylloideae, leaf anatomy, Neotropics, South America, taxonomy. Um novo sinônimo para Micropholis gardneriana (Sapotaceae) com descrição completa, anatomia e notas de distribuição Resumo: No presente trabalho, sinonimizamos Micropholis compta sob M. gardneriana devido à sobreposição de caracteres morfoanatômicos e ausência de atributos distintivos, verificado durante estudo taxonômico e anatômico do gênero Micropholis para o Brasil. -

Gambeya Korupensis (Sapotaceae: Chrysophylloideae), a New Rain Forest Tree Species from the Southwest Region in Cameroon
KEW BULLETIN (2016) 71:28 ISSN: 0075-5974 (print) DOI 10.1007/S12225-016-9633-X ISSN: 1874-933X (electronic) Gambeya korupensis (Sapotaceae: Chrysophylloideae), a new rain forest tree species from the Southwest Region in Cameroon Corneille E. N. Ewango1,2, David Kenfack3, Moses Nsanyi Sainge4, Duncan W. Thomas5 & Xander M. van der Burgt6 Summary. Gambeya korupensis Ewango & Kenfack (Sapotaceae: Chrysophylloideae), a new rain forest tree species from the Southwest Region in Cameroon, is described and illustrated. A distribution map is provided. G. korupensis has the leaf blade below pubescent on the midribs and secondary nerves, flowers with a pedicel 0.5 – 1 mm long, and a fruit which is ovoid, attenuate at the apex, 5-ridged, verrucose between the ridges, and bright red at maturity. The conservation status of G. korupensis is assessed as Vulnerable according to IUCN criteria. Key Words. Chrysophyllum, conservation, IUCN Vulnerable, Korup National Park. Introduction 2006; Burgt 2009; Ewango & Breteler 2001; Kenfack Tropical forests inspire botanists and ecologists et al. 2004). The collections were also compared with because of their high diversity and the numerous authoritatively named material of all tropical African species still to be described. Great interest has been species of Gambeya in various herbaria (mostly still aroused by the likely impact of climate change and stored under Chrysophyllum L.; see below). The species fi development on their species diversity and more effort was identi ed as new and provisionally named as Tulestea is needed to document poorly known areas of sp. nov. based on fruit structure by D. W. Thomas biodiversity conservation priority, before their species (Thomas et al.