A Mineral X-Ray Linear Attenuation Coefficient Tool
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -
Advanced Physics Laboratory
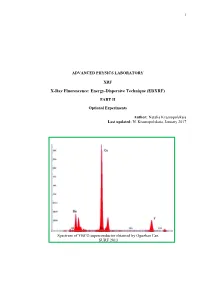
1 ADVANCED PHYSICS LABORATORY XRF X-Ray Fluorescence: Energy-Dispersive Technique (EDXRF) PART II Optional Experiments Author: Natalia Krasnopolskaia Last updated: N. Krasnopolskaia, January 2017 Spectrum of YBCO superconductor obtained by Oguzhan Can. SURF 2013 2 Contents Introduction ………………………………………………………………………………………3 Experiment 1. Quantitative elemental analysis of coins ……………..………………………….12 Experiment 2. Study of Si PIN detector in the x-ray fluorescence spectrometer ……………….16 Experiment 3. Secondary enhancement of x-ray fluorescence and matrix effects in alloys.……24 Experiment 4. Verification of stoichiometry formula for superconducting materials..………….29 3 INTRODUCTION In Part I of the experiment you got familiar with fundamental ideas about the origin of x-ray photons as a result of decelerating of electrons in an x-ray tube and photoelectric effect in atoms of the x-ray tube and a target. For practical purposes, the processes in the x-ray tube and in the sample of interest must be explained in detail. An X-ray photon from the x-ray tube incident on a target/sample can be either absorbed by the atoms of the target or scattered. The corresponding processes are: (a) photoelectric effect (absorption) that gives rise to characteristic spectrum emitted isotropically; (b) Compton effect (inelastic scattering; energy shifts toward the lower value); and (c) Rayleigh scattering (elastic scattering; energy of the photon stays unchanged). Each event of interaction between the incident particle and an atom or the other particle is characterized by its probability, which in spectroscopy is expressed through the cross section σ of the process. Actually, σ has a dimension of [L2] and in atomic and nuclear physics has a unit of barn: 1 barn = 10-24cm2. -

Remote Sensing
remote sensing Article A Refined Four-Stream Radiative Transfer Model for Row-Planted Crops Xu Ma 1, Tiejun Wang 2 and Lei Lu 1,3,* 1 College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] 2 Faculty of Geo-Information Science and Earth Observation (ITC), University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 3 Key Laboratory of Western China’s Environmental Systems (Ministry of Education), Lanzhou University, Lanzhou 730000, China * Correspondence: [email protected]; Tel.: +86-151-1721-8663 Received: 17 March 2020; Accepted: 14 April 2020; Published: 18 April 2020 Abstract: In modeling the canopy reflectance of row-planted crops, neglecting horizontal radiative transfer may lead to an inaccurate representation of vegetation energy balance and further cause uncertainty in the simulation of canopy reflectance at larger viewing zenith angles. To reduce this systematic deviation, here we refined the four-stream radiative transfer equations by considering horizontal radiation through the lateral “walls”, considered the radiative transfer between rows, then proposed a modified four-stream (MFS) radiative transfer model using single and multiple scattering. We validated the MFS model using both computer simulations and in situ measurements, and found that the MFS model can be used to simulate crop canopy reflectance at different growth stages with an accuracy comparable to the computer simulations (RMSE < 0.002 in the red band, RMSE < 0.019 in NIR band). Moreover, the MFS model can be successfully used to simulate the reflectance of continuous (RMSE = 0.012) and row crop canopies (RMSE < 0.023), and therefore addressed the large viewing zenith angle problems in the previous row model based on four-stream radiative transfer equations. -

The Optical Effective Attenuation Coefficient As an Informative
hv photonics Article The Optical Effective Attenuation Coefficient as an Informative Measure of Brain Health in Aging Antonio M. Chiarelli 1,2,* , Kathy A. Low 1, Edward L. Maclin 1, Mark A. Fletcher 1, Tania S. Kong 1,3, Benjamin Zimmerman 1, Chin Hong Tan 1,4,5 , Bradley P. Sutton 1,6, Monica Fabiani 1,3,* and Gabriele Gratton 1,3,* 1 Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 2 Department of Neuroscience, Imaging and Clinical Sciences, University G. D’Annunzio of Chieti-Pescara, 66100 Chieti, Italy 3 Psychology Department, University of Illinois at Urbana-Champaign, Champaign, IL 61820, USA 4 Division of Psychology, Nanyang Technological University, Singapore 639818, Singapore 5 Department of Pharmacology, National University of Singapore, Singapore 117600, Singapore 6 Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA * Correspondence: [email protected] (A.M.C.); [email protected] (M.F.); [email protected] (G.G.) Received: 24 May 2019; Accepted: 10 July 2019; Published: 12 July 2019 Abstract: Aging is accompanied by widespread changes in brain tissue. Here, we hypothesized that head tissue opacity to near-infrared light provides information about the health status of the brain’s cortical mantle. In diffusive media such as the head, opacity is quantified through the Effective Attenuation Coefficient (EAC), which is proportional to the geometric mean of the absorption and reduced scattering coefficients. EAC is estimated by the slope of the relationship between source–detector distance and the logarithm of the amount of light reaching the detector (optical density). -

Gamma-Ray Interactions with Matter
2 Gamma-Ray Interactions with Matter G. Nelson and D. ReWy 2.1 INTRODUCTION A knowledge of gamma-ray interactions is important to the nondestructive assayist in order to understand gamma-ray detection and attenuation. A gamma ray must interact with a detector in order to be “seen.” Although the major isotopes of uranium and plutonium emit gamma rays at fixed energies and rates, the gamma-ray intensity measured outside a sample is always attenuated because of gamma-ray interactions with the sample. This attenuation must be carefully considered when using gamma-ray NDA instruments. This chapter discusses the exponential attenuation of gamma rays in bulk mater- ials and describes the major gamma-ray interactions, gamma-ray shielding, filtering, and collimation. The treatment given here is necessarily brief. For a more detailed discussion, see Refs. 1 and 2. 2.2 EXPONENTIAL A~ATION Gamma rays were first identified in 1900 by Becquerel and VMard as a component of the radiation from uranium and radium that had much higher penetrability than alpha and beta particles. In 1909, Soddy and Russell found that gamma-ray attenuation followed an exponential law and that the ratio of the attenuation coefficient to the density of the attenuating material was nearly constant for all materials. 2.2.1 The Fundamental Law of Gamma-Ray Attenuation Figure 2.1 illustrates a simple attenuation experiment. When gamma radiation of intensity IO is incident on an absorber of thickness L, the emerging intensity (I) transmitted by the absorber is given by the exponential expression (2-i) 27 28 G. -

Glossary Radiation Physics Phase
Glossary Radiation Physics Phase space co-ordinates : (x,y,z,E,θ,φ), where (x,y,z) are the Cartesian coordinates of the point in configuration space where the field is evaluated, E is the particle (generically including photons) energy, and (θ,φ) are the spherical polar coordinates describing the particle direction of motion. Condensed form of phase space coordinates: (r,E) where r'xLx%yLy%zLz and E=EΩ. The vector Ω'sinθcosφLx%sinθsinφLy%cosθLz , and is a unit vector pointing in the direction of the particle velocity. Phase space volume element: d 3rd 3E'd 3rdEdΩ'dxdydzdEsinθdθdφ (cm 3&MeV&Sr) Total number of particles or photons at time t: N(t) Angular number density spectrum: d 6N n˜(r,E,t)' (cm &3&Mev &1&Sr &1) d 3rd 3E Number density spectrum: n(r,E,t)' n˜(r,E,t)dΩ m 4π Angular fluence rate spectrum: d 6N n˜ (r,E,t)'n˜(r,E,t)v' (cm &2&MeV &1&Sr &1&s &1) 3 dAzd Edt 3 where (d r'dAzvdt) Also known as angular flux density spectrum. Fluence rate spectrum: d 4N n(r,E,t)' n˜ (r,E,t)dΩ' (cm &2&MeV &1&s &1) m dAdEdt 4π Where dA is the cross sectional area of sphere centered at r Vector Fluence rate spectrum or current density: J(r,E,t)' n˜ (r,E,t)ΩdΩ (cm &2&MeV &1&s &1) m 4π d 4N J(r,E,t)@ν' Where dA is an elemental area with normal ν dAdEdt Angular fluence spectrum: t Φ˜ (r,E)' φ˜(r,E,tN)dtN (cm &2&MeV &1&Sr &1) m 0 Where t is the total exposure duration Fluence Spectrum: Φ(r,E)' Φ˜ (r,E)dΩ (cm &2&MeV &1) m 4π Energy flow vector: g(r,t)' φ˜(r,E,t)Ed 3E (MeV&cm &2&s &1) m Spectral radiance: The radiant flux per unit cross sectional area per unit solid angle per unit wave length 6 d R &2 &1 &1 Lλ(r,Ω,λ,t)' (W&m Sr nm ) dAzdΩdλ Spectral irradiance: The radiant flux per unit surface area per unit wavelength: E (r,λ,t)' L (r,Ω,λ,t)cosθdΩ (W&m &2nm &1) λ m λ Linear attenuation coefficient: The probability per unit particle path length for an interaction with the medium in which it is propagating. -

Model of the Attenuation Coefficient of Daily Photosynthetically Available
Methods in Oceanography 8 (2013) 56–74 Contents lists available at ScienceDirect Methods in Oceanography journal homepage: www.elsevier.com/locate/mio Full length article Model of the attenuation coefficient of daily photosynthetically available radiation in the upper ocean Jianwei Wei ∗, ZhongPing Lee School for the Environment, University of Massachusetts Boston, 100 Morrissey Blvd., Boston, MA 02125, USA article info a b s t r a c t Article history: Penetration of the photosynthetically available radiation (PAR, over Available online 17 December 2013 400–700 nm) in the upper ocean is important for many processes such as water radiant heating and primary productivity. Because Keywords: of this importance, daily PAR at sea surface (PAR.0C/) is routinely Photosynthetically available radiation generated from ocean-color images for global studies. To propa- Diffuse attenuation coefficient Diurnal variability gate this broadband solar radiation through the upper ocean, an Inherent optical properties attenuation coefficient of PAR (KPAR) is also generated from the same ocean-color measurements. However, due to the empirical nature of the KPAR algorithm, this KPAR product corresponds to an instantaneous PAR at a fixed sun angle, with no diurnal variability. It is hence necessary to have an attenuation coefficient matching the temporal characteristics of daily PAR. This paper represents an effort to meet this need. Using ECOLIGHT, the subsurface light field for a wide range of water bodies was simulated, from which the attenuation coefficient (KPAR) of daily PAR was calculated. We pre- sented the diurnal and vertical variation of this attenuation coef- ficient, and found that it can be well predicted (within ∼7%) as a function of the total absorption coefficient and backscattering coefficient at 490 nm and the noontime solar zenith angle. -

Spaceborn Optoelectronic Sensors and Their Radiometric Calibration
Spaceborne Optoelectronic Sensors and their Radiometric Calibration. Terms and Definitions. Part 1. Calibration Techniques Alexander V. Prokhorov Raju U. Datla Vitaly P. Zakharenkov Victor Privalsky Thomas W. Humpherys Victor I. Sapritsky Editors: Albert C. Parr Lev K. Issaev QC NIST too National Institute of Standards and Technology *U,SL Technology Administration, U.S. Department of Commerce * 7*03 *oo 5 NISTIR 7203 Spaceborne Optoelectronic Sensors and their Radiometric Calibration. Terms and Definitions. Part 1. Calibration Techniques Alexander V. Prokhorov Raju U. Datla Vitaly P. Zakharenkov Victor Privalsky Thomas W. Humpherys Victor I. Sapritsky Editors: Albert C. Parr Lev K. Issaev NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NISTIR 7203 Spaceborne Optoelectronic Sensors and their Radiometric Calibration. Terms and Definitions Part 1. Calibration Techniques Alexander V. Prokhorov Raju U. Datla NIST Vitaly P. Zakharenkov Vavilov State Optical Insitute, S. -Petersburg, Russia Victor Privalsky Thomas W. Humpherys Space Dynamics Laboratory/Utah State University, Logan, UT Victor I. Sapritsky All-Russian Research Institute for Optical and Physical Measurements, Moscow, Russia Editors: Albert C. Parr NIST Lev K. Issaev Russian Institute ofMetrological Seiwice, Moscow, Russia March 2005 U.S. DEPARTMENT OF COMMERCE Carlos M. Gutierrez, Secretary TECHNOLOGY ADMINISTRATION Phillip J. Bond, Under Secretaiy of Commercefor Technology' NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY Hratch G. Semerjian, Acting Director 0imHHKMICKTPOHHbl8 flaT4MKM KOCMMMeCKOlO I83MIMBMNI M MX paAMOMeTPHiecKafl KanuGpoBKa TepMHHbi h onpe^ejieHHH HacTb 1. MeTO/jbi Kajm6poBKH AjieKcaimp IlpoxopoB, Pa^y /JaTJia, HaijHOHajibHbiH HHCTHTyT CTaH^apTOB h TexHOJiorHH, reHTec6epr, niTaT MepHnenr, CUJA BMTajiHH 3axapeHKOB, rocy^apcTBeHHbm ohthhcckhh HHCTHTyT hm. C. -

Basic Health Physics
Shielding Radiation Alphas, Betas, Gammas and Neutrons 7/05/2011 1 Contents General General Shielding Alpha Emitters Shielding Alpha Emitters Shie lding Be ta Par tic les General Beta Particle Range Bremsstrahlung Example Calculation Shielding Positrons Shielding Positrons 2 Contents Shielding Gamma Rays General Basic Equation – First Example Calculation Basic Equation – Second Example Calculation Relaxation Length Calculating the Required Shielding Thickness Calculating the Required Shielding Thickness – Example Shielding Multiple Gamma Ray Energies Shielding Multiple Gamma Ray Energies – Example Layered (Compound) Shielding Layered (Compound) Shielding – Example Half Value Layers Half Value layers – Example 3 Contents Shielding Neutrons continued Multipurpose Materials for neutron Shields Possible Neutron Shield Options Neutron Shielding Calculations – Fast Neutrons Neutron Shielding Calculations – Alpha-Beryllium Sources Neutron Shielding Calculations Gamma and Neutron Shields – General General Radiation energy Shielding Material Shielding Material - Lead 4 Contents Shielding Gamma Rays continued Tenth Value layers Buildup Buildup Factor Buildup Factor Example Determining the Required shield Thickness with Buildup Shielding Neutrons The Three Steps 1. Slow the Neutrons 2. Absorb the Neutrons 3. Absorb the Gamma rays 5 Contents Gamma and Neutron Shields – General continued Shielding Material – Water Shielding Material – Concrete Streaming Streaming – Door Design Streaming – Labyrinth Shine Appendices Appendix A – Densities of Common -

Attenuation of Radiation in Matter in This Experiment We Will Examine
Attenuation of Radiation in Matter In this experiment we will examine how radiation decreases in intensity as it passes through a substance. Since radiation interacts with matter, its intensity will decrease as it travels through a material. The attenuation properties of radiation will effect how much shielding is necessary and how much dosage one recieves. Since radiation deposits its energy as it travels though matter, the amount of dosage one receives depends on how the radiation is attenuated. Radiation that attenuates fast, i.e. loses its energy in a short distance, will give a higher dosage (near the surface) than radiation that attenuates slowly, i.e. loses its energy over a long distance in the material. First we consider how the intensity of gamma particles decreases as they pass through a material. We will see that the attenuation properties of gamma particles can be described fairly simply. The attenuation of β and α particles is more complicated. Attenuation of gamma particles As gamma particles pass through matter, they interact with the material and get absorbed. Suppose gamma particles are incident from the left on a slab, and the slab has a thickness of x. After passing through the slab of material, the gamma particles emerge on the right. Let the intensity of the gammas incident from the left be denoted as I0, the initial intensity. Let the intensity of the gammas that emerge on the right, after passing though the slab, be denoted as I(x), the final intensity. The unit for intensity, I0 and I(x), is (number of gamma particles)/area/time. -

A Theorettcal Approach to the Attenuation Coefficient of Light in Sea Water
A THEORETTCAL APPROACH TO THE ATTENUATION COEFFICIENT OF LIGHT IN SEA WATER A.V.S. MURTY Central Marine Fisheries Research Institute, Cochin-ll ABSTRACT The attenuation coeflScient of sea water is derived from a formula which is more general than the Lambert's equation. The mean attenuation coefficient which the Secchi disc observations give is obtained therefrom. The constant of the Secchi disc is discussed in the light of the relationship developed. The theoretical concept developed here, falls in accordance with the expressions based on field data. INTRODUCTION The attenuation coefficient of light in sea water is an important parameter not only from its physical point of view but also from the view point of its relationship to primary production (Riley, 1956 and Strickland, 1958). The attenuation coeffi cient of light depends upon absorption and scattering in the medium. The absorp tion and scattering caused by. sea water at different wavelengths of light have been discussed in detail by Jerlov (1967). Although modern electronic devices (Gall, 1949; Jerlov, 1951 and Sasaki, 1964) are available to determine quite accurately the attenuation coefficients, the Seccbi disc is still the simplest device to determine quite easily the mean attenuation coeffi cient of sea water for visible radiation. Though the device is simple, a theoretical approach toVvards its perfection makes it a more practical object for use. Tyler (1968) developed a fine theory on Secchi disc. His aim was, however, to divide the attenuation coefficient as determined by Secchi disc into its primary components, namely, the two coefficients for coUimated and diffused light. Certain suggestions have appeared in modern literature on the use of Secchi disc (Jones and Wills, 1956; Hanaoka, 1957 and Qasim, Bhattathiri and Abidi, 1968) with some modifications in the expression originally determined by Poole and Atkins (1929) based on their studies in the English Channel.