Attenuation of Radiation in Matter in This Experiment We Will Examine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -

Seasonal and Spatial Variations in the Attenuation of Light in the North Atlantic Ocean
JEFFREY H. SMART SEASONAL AND SPATIAL VARIATIONS IN THE ATTENUATION OF LIGHT IN THE NORTH ATLANTIC OCEAN This article examines seasonal and spatial vanatlOns in the average depth profiles of the diffuse attenuation coefficient, Kd, for visible light propagating downward from the ocean surface at wavelengths of 490 and 532 nm. Profiles of the depth of the nth attenuation length are also shown. Spring, summer, and fall data from the northwestern and north central Atlantic Ocean and winter data from the northeastern Atlantic Ocean are studied. Variations in the depth-integrated Kd profiles are important because they are related to the depth penetration achievable by an airborne active optical antisubmarine warfare system. INTRODUCTION Numerous investigators have tried to characterize the the geographic areas and seasons indicated in Table 1 and diffuse attenuation coefficient (hereafter denoted as Kd) Figure 1. Despite the incomplete spatial and temporal for various ocean areas and for the different seasons. Platt coverage across the North Atlantic Ocean, this article and Sathyendranath 1 generated numerical fits to averaged should serve as a useful resource to estimate the optical profiles from coastal and open-ocean regions, and they characteristics in the areas studied. divided the North Atlantic into provinces that extended The purpose of this article is to characterize the sea from North America to Europe and over large ranges sonal and spatial variations in diffuse attenuation at 490 in latitude (e.g. , from 10.0° to 37.00 N and from 37.0° to and 532 nm. Because Kd frequently changes with depth, 50.00 N). In addition, numerous papers have attempted profiles of depth versus number of attenuation lengths are to model chlorophyll concentrations (which are closely required to characterize the attenuation of light as it correlated with Kd ) using data on available sunlight as a propagates from the surface downward into the water function of latitude and season, nutrient concentrations, column. -

Natural and Enhanced Attenuation of Soil and Ground Water At
LMS/MON/S04243 Office of Legacy Management Natural and Enhanced Attenuation of Soil and Groundwater at Monument Valley, Arizona, and Shiprock, New Mexico, DOE Legacy Waste Sites 2007 Pilot Study Status Report June 2008 U.S. Department OfficeOffice ofof LegacyLegacy ManagementManagement of Energy Work Performed Under DOE Contract No. DE–AM01–07LM00060 for the U.S. Department of Energy Office of Legacy Management. Approved for public release; distribution is unlimited. This page intentionally left blank LMS/MON/S04243 Natural and Enhanced Attenuation of Soil and Groundwater at Monument Valley, Arizona, and Shiprock, New Mexico, DOE Legacy Waste Sites 2007 Pilot Study Status Report June 2008 This page intentionally left blank Contents Acronyms and Abbreviations ....................................................................................................... vii Executive Summary....................................................................................................................... ix 1.0 Introduction......................................................................................................................1–1 2.0 Monument Valley Pilot Studies.......................................................................................2–1 2.1 Source Containment and Removal..........................................................................2–1 2.1.1 Phreatophyte Growth and Total Nitrogen...................................................2–1 2.1.2 Causes and Recourses for Stunted Plant Growth........................................2–5 -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Atmosphere Observation by the Method of LED Sun Photometry
Atmosphere Observation by the Method of LED Sun Photometry A Senior Project presented to the Faculty of the Physics Department California Polytechnic State University, San Luis Obispo In Partial Fulfillment of the Requirements of the Degree Bachelor of Science by Gregory Garza April 2013 1 Introduction The focus of this project is centered on the subject of sun photometry. The goal of the experiment was to use a simple self constructed sun photometer to observe how attenuation coefficients change over longer periods of time as well as the determination of the solar extraterrestrial constants for particular wavelengths of light. This was achieved by measuring changes in sun radiance at a particular location for a few hours a day and then use of the Langley extrapolation method on the resulting sun radiance data set. Sun photometry itself is generally involved in the practice of measuring atmospheric aerosols and water vapor. Roughly a century ago, the Smithsonian Institutes Astrophysical Observatory developed a method of measuring solar radiance using spectrometers; however, these were not usable in a simple hand-held setting. In the 1950’s Frederick Volz developed the first hand-held sun photometer, which he improved until coming to the use of silicon photodiodes to produce a photocurrent. These early stages of the development of sun photometry began with the use of silicon photodiodes in conjunction with light filters to measure particular wavelengths of sunlight. However, this method of sun photometry came with cost issues as well as unreliability resulting from degradation and wear on photodiodes. A more cost effective method was devised by amateur scientist Forrest Mims in 1989 that incorporated the use of light emitting diodes, or LEDs, that are responsive only to the light wavelength that they emit. -

Radio Astronomy
Edition of 2013 HANDBOOK ON RADIO ASTRONOMY International Telecommunication Union Sales and Marketing Division Place des Nations *38650* CH-1211 Geneva 20 Switzerland Fax: +41 22 730 5194 Printed in Switzerland Tel.: +41 22 730 6141 Geneva, 2013 E-mail: [email protected] ISBN: 978-92-61-14481-4 Edition of 2013 Web: www.itu.int/publications Photo credit: ATCA David Smyth HANDBOOK ON RADIO ASTRONOMY Radiocommunication Bureau Handbook on Radio Astronomy Third Edition EDITION OF 2013 RADIOCOMMUNICATION BUREAU Cover photo: Six identical 22-m antennas make up CSIRO's Australia Telescope Compact Array, an earth-rotation synthesis telescope located at the Paul Wild Observatory. Credit: David Smyth. ITU 2013 All rights reserved. No part of this publication may be reproduced, by any means whatsoever, without the prior written permission of ITU. - iii - Introduction to the third edition by the Chairman of ITU-R Working Party 7D (Radio Astronomy) It is an honour and privilege to present the third edition of the Handbook – Radio Astronomy, and I do so with great pleasure. The Handbook is not intended as a source book on radio astronomy, but is concerned principally with those aspects of radio astronomy that are relevant to frequency coordination, that is, the management of radio spectrum usage in order to minimize interference between radiocommunication services. Radio astronomy does not involve the transmission of radiowaves in the frequency bands allocated for its operation, and cannot cause harmful interference to other services. On the other hand, the received cosmic signals are usually extremely weak, and transmissions of other services can interfere with such signals. -
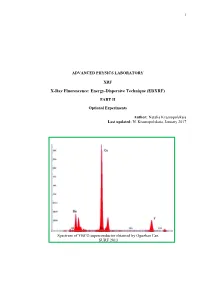
Advanced Physics Laboratory
1 ADVANCED PHYSICS LABORATORY XRF X-Ray Fluorescence: Energy-Dispersive Technique (EDXRF) PART II Optional Experiments Author: Natalia Krasnopolskaia Last updated: N. Krasnopolskaia, January 2017 Spectrum of YBCO superconductor obtained by Oguzhan Can. SURF 2013 2 Contents Introduction ………………………………………………………………………………………3 Experiment 1. Quantitative elemental analysis of coins ……………..………………………….12 Experiment 2. Study of Si PIN detector in the x-ray fluorescence spectrometer ……………….16 Experiment 3. Secondary enhancement of x-ray fluorescence and matrix effects in alloys.……24 Experiment 4. Verification of stoichiometry formula for superconducting materials..………….29 3 INTRODUCTION In Part I of the experiment you got familiar with fundamental ideas about the origin of x-ray photons as a result of decelerating of electrons in an x-ray tube and photoelectric effect in atoms of the x-ray tube and a target. For practical purposes, the processes in the x-ray tube and in the sample of interest must be explained in detail. An X-ray photon from the x-ray tube incident on a target/sample can be either absorbed by the atoms of the target or scattered. The corresponding processes are: (a) photoelectric effect (absorption) that gives rise to characteristic spectrum emitted isotropically; (b) Compton effect (inelastic scattering; energy shifts toward the lower value); and (c) Rayleigh scattering (elastic scattering; energy of the photon stays unchanged). Each event of interaction between the incident particle and an atom or the other particle is characterized by its probability, which in spectroscopy is expressed through the cross section σ of the process. Actually, σ has a dimension of [L2] and in atomic and nuclear physics has a unit of barn: 1 barn = 10-24cm2. -

Remote Sensing
remote sensing Article A Refined Four-Stream Radiative Transfer Model for Row-Planted Crops Xu Ma 1, Tiejun Wang 2 and Lei Lu 1,3,* 1 College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] 2 Faculty of Geo-Information Science and Earth Observation (ITC), University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 3 Key Laboratory of Western China’s Environmental Systems (Ministry of Education), Lanzhou University, Lanzhou 730000, China * Correspondence: [email protected]; Tel.: +86-151-1721-8663 Received: 17 March 2020; Accepted: 14 April 2020; Published: 18 April 2020 Abstract: In modeling the canopy reflectance of row-planted crops, neglecting horizontal radiative transfer may lead to an inaccurate representation of vegetation energy balance and further cause uncertainty in the simulation of canopy reflectance at larger viewing zenith angles. To reduce this systematic deviation, here we refined the four-stream radiative transfer equations by considering horizontal radiation through the lateral “walls”, considered the radiative transfer between rows, then proposed a modified four-stream (MFS) radiative transfer model using single and multiple scattering. We validated the MFS model using both computer simulations and in situ measurements, and found that the MFS model can be used to simulate crop canopy reflectance at different growth stages with an accuracy comparable to the computer simulations (RMSE < 0.002 in the red band, RMSE < 0.019 in NIR band). Moreover, the MFS model can be successfully used to simulate the reflectance of continuous (RMSE = 0.012) and row crop canopies (RMSE < 0.023), and therefore addressed the large viewing zenith angle problems in the previous row model based on four-stream radiative transfer equations. -

The Optical Effective Attenuation Coefficient As an Informative
hv photonics Article The Optical Effective Attenuation Coefficient as an Informative Measure of Brain Health in Aging Antonio M. Chiarelli 1,2,* , Kathy A. Low 1, Edward L. Maclin 1, Mark A. Fletcher 1, Tania S. Kong 1,3, Benjamin Zimmerman 1, Chin Hong Tan 1,4,5 , Bradley P. Sutton 1,6, Monica Fabiani 1,3,* and Gabriele Gratton 1,3,* 1 Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 2 Department of Neuroscience, Imaging and Clinical Sciences, University G. D’Annunzio of Chieti-Pescara, 66100 Chieti, Italy 3 Psychology Department, University of Illinois at Urbana-Champaign, Champaign, IL 61820, USA 4 Division of Psychology, Nanyang Technological University, Singapore 639818, Singapore 5 Department of Pharmacology, National University of Singapore, Singapore 117600, Singapore 6 Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA * Correspondence: [email protected] (A.M.C.); [email protected] (M.F.); [email protected] (G.G.) Received: 24 May 2019; Accepted: 10 July 2019; Published: 12 July 2019 Abstract: Aging is accompanied by widespread changes in brain tissue. Here, we hypothesized that head tissue opacity to near-infrared light provides information about the health status of the brain’s cortical mantle. In diffusive media such as the head, opacity is quantified through the Effective Attenuation Coefficient (EAC), which is proportional to the geometric mean of the absorption and reduced scattering coefficients. EAC is estimated by the slope of the relationship between source–detector distance and the logarithm of the amount of light reaching the detector (optical density). -

Causes of Light Attenuation with Respect to Seagrasses in Upper and Lower Charlotte Harbor
Causes of Light Attenuation with Respect to Seagrasses in Upper and Lower Charlotte Harbor Final Report Submitted to: Southwest Florida Water Management District Surface Water Improvement and Management Program 7601 U.S. Highway 301 North Tampa, FL 33637 (813) 985-7481 Charlotte Harbor National Estuary Program 4980 Bayline Drive 4th Floor North Fort Myers, Florida 33917 (941) 995-1777 Submitted by: L. Kellie Dixon and Gary J. Kirkpatrick Mote Marine Laboratory 1600 Ken Thompson Parkway Sarasota, FL 34236 (941) 388-4441 September 20, 1999 Mote Marine Laboratory Technical Report No. 650. Please note that pages do not always correspond to those listed in the table of contents. This was caused when the document was converted to a PDF file. Acknowledgments Thanks are due to many, but especially Linda Franklin, Jay Leverone, Tiffany Lutterman, Ari Nissanka, Jon Perry, Joie Simoneaux, Roberta Starke, and David Tomasko. i Table of Contents Acknowledgments.............................................................................................................................i Table of Contents.............................................................................................................................ii List of Tables...................................................................................................................................iv List of Figures...................................................................................................................................v Executive Summary.........................................................................................................................ix -

Mass Attenuation Coefficients of X-Rays in Different Barite Concrete Used in Radiation Protection As Shielding Against Ionizing Radiation
X congreso Regional Latinoamericano IRPA de Protección y Seguridad Radiológica Radi opro te ce ion: Nuevos Desafíos para un Mundo en Evolución ” Buenos Aires, 12 al 17 de abril, 2015 SOCIEDAD ARGENTINA DE RADIOPROTECCIÓN MASS ATTENUATION COEFFICIENTS OF X-RAYS IN DIFFERENT BARITE CONCRETE USED IN RADIATION PROTECTION AS SHIELDING AGAINST IONIZING RADIATION Almeida Junior, Airton T.1’4’5, Nogueira, M. S.2, Santos, M. A. P3, L. L. Campos 4, Araújo, F. G. S.5, 1 Brazilian Institute for Safety and Health at Work - FUNDACENTRO, Minas Gerais, Brazil 2 Center of Development of Nuclear Technology - CDTN/CNEN, Minas Gerais, Brazil 3 Regional Center for Nuclear Science - CRCN/CNEN, Pernambuco, Brazil 4 Institute of Energy and Nuclear Research - IPEN/CNEN, São Paulo, Brazil 5 Universidade Federal de Ouro Preto - UFOP /REDEMAT, Minas Gerais, Brazil ABSTRACT The attenuation coefficient depends on the incident photon energy and the nature of the materials. In order to minimize exposure to individuals. Barite concrete has been largely used as a shielding material in installations housing gamma radiation sources as well as X-ray generating equipment. This study was conducted to evaluate the efficacy of different mixtures of barite concrete for shielding in diagnostic X-ray rooms. The mass attenuation coefficient (jn/p). The mass attenuation coefficients have been measured by employing the CdTe detector model XR-1OOT. The distance between the source and the exposed surface of all samples was measured by SSD light indicator of machine which was 350 cm. The slope of the linear plot of the intensity transmitted versus specimen thickness would yield the attenuation coefficient. -
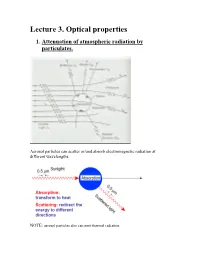
Lecture 3. Optical Properties 1
Lecture 3. Optical properties 1. Attenuation of atmospheric radiation by particulates. Aerosol particles can scatter or/and absorb electromagnetic radiation at different wavelengths. NOTE: aerosol particles also can emit thermal radiation. Scattering is a process, which conserves the total amount of energy, but the direction in which the radiation propagates may be altered. Absorption is a process that removes energy from the electromagnetic radiation field, and converts it to another form. Extinction (or attenuation) is the sum of scattering and absorption, so it represents total effect of medium on radiation passing the medium. In the atmosphere: aerosol particles can scatter and absorb solar and infrared radiation altering air temperature and the rates of photochemical reactions. Key parameters that govern the scattering and absorption of radiation by a particle: i. the wavelength of the incident radiation; ii. the size of the particles, expressed as a dimensional size parameter x: D x (where D is the particle diameter); iii. complex refractive index (or optical constant) of a particle: m = n + i k where n is the real part of the refractive index, k is the imaginary part of the refractive index. Both n and k depend on the wavelength. Important to remember: complex refractive index of a particle is defined by its chemical composition; real part , n , is responsible for scattering. imaginary part, k, is responsible for absorption. If k is equal to 0 at a given wavelength thus a particle does not absorb radiation at this wavelength. Table 3.1 Some refractive indices of atmospheric aerosol substances at = 0.5 m.