Inflammation and Wound Healing: the Role of the Macrophage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Infection and Inflammation on Biomarkers of Nutritional Status
A2.4 INFLUENCE OF INFECTION AND INFLAMMATION ON BIOMARKERS OF NUTRITIONAL STATUS A2.4 Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron David I. Thurnham1 and George P. McCabe2 1 Northern Ireland Centre for Food and Health, University of Ulster, Coleraine, United Kingdom of Great Britain and Northern Ireland 2 Statistics Department, Purdue University, West Lafayette, Indiana, United States of America Corresponding author: David I. Thurnham; [email protected] Suggested citation: Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva, World Health Organization, 2012. Abstract n Many plasma nutrients are influenced by infection or tissue damage. These effects may be passive and the result of changes in blood volume and capillary permeability. They may also be the direct effect of metabolic alterations that depress or increase the concentration of a nutrient or metabolite in the plasma. Where the nutrient or metabolite is a nutritional biomarker as in the case of plasma retinol, a depression in retinol concentrations will result in an overestimate of vitamin A deficiency. In contrast, where the biomarker is increased due to infection as in the case of plasma ferritin concentrations, inflammation will result in an underestimate of iron deficiency. Infection and tissue damage can be recognized by their clinical effects on the body but, unfortunately, subclinical infection or inflammation can only be recognized by measur- ing inflammation biomarkers in the blood. -

The Gut Microbiota and Inflammation
International Journal of Environmental Research and Public Health Review The Gut Microbiota and Inflammation: An Overview 1, 2 1, 1, , Zahraa Al Bander *, Marloes Dekker Nitert , Aya Mousa y and Negar Naderpoor * y 1 Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Melbourne 3168, Australia; [email protected] 2 School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane 4072, Australia; [email protected] * Correspondence: [email protected] (Z.A.B.); [email protected] (N.N.); Tel.: +61-38-572-2896 (N.N.) These authors contributed equally to this work. y Received: 10 September 2020; Accepted: 15 October 2020; Published: 19 October 2020 Abstract: The gut microbiota encompasses a diverse community of bacteria that carry out various functions influencing the overall health of the host. These comprise nutrient metabolism, immune system regulation and natural defence against infection. The presence of certain bacteria is associated with inflammatory molecules that may bring about inflammation in various body tissues. Inflammation underlies many chronic multisystem conditions including obesity, atherosclerosis, type 2 diabetes mellitus and inflammatory bowel disease. Inflammation may be triggered by structural components of the bacteria which can result in a cascade of inflammatory pathways involving interleukins and other cytokines. Similarly, by-products of metabolic processes in bacteria, including some short-chain fatty acids, can play a role in inhibiting inflammatory processes. In this review, we aimed to provide an overview of the relationship between the gut microbiota and inflammatory molecules and to highlight relevant knowledge gaps in this field. -

Energy Healing
57618_CH03_Pass2.QXD 10/30/08 1:19 PM Page 61 © Jones and Bartlett Publishers, LLC. NOT FOR SALE OR DISTRIBUTION. CHAPTER 3 Energy Healing Our remedies oft in ourselves do lie. —WILLIAM SHAKESPEARE LEARNING OBJECTIVES 1. Describe the types of energy. 2. Explain the universal energy field (UEF). 3. Explain the human energy field (HEF). 4. Describe the seven auric layers. 5. Describe the seven chakras. 6. Define the concept of energy healing. 7. Describe various types of energy healing. INTRODUCTION For centuries, traditional healers worldwide have practiced methods of energy healing, viewing the body as a complex energy system with energy flowing through or over its surface (Rakel, 2007). Until recently, the Western world largely ignored the Eastern interpretation of humans as energy beings. However, times have changed dramatically and an exciting and promising new branch of academic inquiry and clinical research is opening in the area of energy healing (Oschman, 2000; Trivieri & Anderson, 2002). Scientists and energy therapists around the world have made discoveries that will forever alter our picture of human energetics. The National Institutes of Health (NIH) is conducting research in areas such as energy healing and prayer, and major U.S. academic institutions are conducting large clinical trials in these areas. Approaches in exploring the concepts of life force and healing energy that previously appeared to compete or conflict have now been found to support each other. Conner and Koithan (2006) note 61 57618_CH03_Pass2.QXD 10/30/08 1:19 PM Page 62 © Jones and Bartlett Publishers, LLC. NOT FOR SALE OR DISTRIBUTION. 62 CHAPTER 3 • ENERGY HEALING that “with increased recognition and federal funding for energetic healing, there is a growing body of research that supports the use of energetic healing interventions with patients” (p. -

Purinergic Signalling in Skin
PURINERGIC SIGNALLING IN SKIN AINA VH GREIG MA FRCS Autonomic Neuroscience Institute Royal Free and University College School of Medicine Rowland Hill Street Hampstead London NW3 2PF in the Department of Anatomy and Developmental Biology University College London Gower Street London WCIE 6BT 2002 Thesis Submitted for the Degree of Doctor of Philosophy University of London ProQuest Number: U643205 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U643205 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT Purinergic receptors, which bind ATP, are expressed on human cutaneous kératinocytes. Previous work in rat epidermis suggested functional roles of purinergic receptors in the regulation of proliferation, differentiation and apoptosis, for example P2X5 receptors were expressed on kératinocytes undergoing proliferation and differentiation, while P2X? receptors were associated with apoptosis. In this thesis, the aim was to investigate the expression of purinergic receptors in human normal and pathological skin, where the balance between these processes is changed. A study was made of the expression of purinergic receptor subtypes in human adult and fetal skin. -

Wound Classification
Wound Classification Presented by Dr. Karen Zulkowski, D.N.S., RN Montana State University Welcome! Thank you for joining this webinar about how to assess and measure a wound. 2 A Little About Myself… • Associate professor at Montana State University • Executive editor of the Journal of the World Council of Enterstomal Therapists (JWCET) and WCET International Ostomy Guidelines (2014) • Editorial board member of Ostomy Wound Management and Advances in Skin and Wound Care • Legal consultant • Former NPUAP board member 3 Today We Will Talk About • How to assess a wound • How to measure a wound Please make a note of your questions. Your Quality Improvement (QI) Specialists will follow up with you after this webinar to address them. 4 Assessing and Measuring Wounds • You completed a skin assessment and found a wound. • Now you need to determine what type of wound you found. • If it is a pressure ulcer, you need to determine the stage. 5 Assessing and Measuring Wounds This is important because— • Each type of wound has a different etiology. • Treatment may be very different. However— • Not all wounds are clear cut. • The cause may be multifactoral. 6 Types of Wounds • Vascular (arterial, venous, and mixed) • Neuropathic (diabetic) • Moisture-associated dermatitis • Skin tear • Pressure ulcer 7 Mixed Etiologies Many wounds have mixed etiologies. • There may be both venous and arterial insufficiency. • There may be diabetes and pressure characteristics. 8 Moisture-Associated Skin Damage • Also called perineal dermatitis, diaper rash, incontinence-associated dermatitis (often confused with pressure ulcers) • An inflammation of the skin in the perineal area, on and between the buttocks, into the skin folds, and down the inner thighs • Scaling of the skin with papule and vesicle formation: – These may open, with “weeping” of the skin, which exacerbates skin damage. -

Wound Care: the Basics
Wound Care: The Basics Suzann Williams-Rosenthal, RN, MSN, WOC, GNP Norma Branham, RN, MSN, WOC, GNP University of Virginia May, 2010 What Type of Wound is it? How long has it been there? Acute-generally heal in a couple weeks, but can become chronic: Surgical Trauma Chronic -do not heal by normal repair process-takes weeks to months: Vascular-venous stasis, arterial ulcers Pressure ulcers Diabetic foot ulcers (neuropathic) Chronic Wounds Pressure Ulcer Staging Where is it? Where is it located? Use anatomical location-heel, ankle, sacrum, coccyx, etc. Measurements-in centimeters Length X Width X Depth • Length = greatest length (head to toe) • Width = greatest width (side to side) • Depth = measure by marking the depth with a Q- Tip and then hold to a ruler Wound Characteristics: Describe by percentage of each type of tissue: Granulation tissue: • red, cobblestone appearance (healing, filling in) Necrotic: • Slough-yellow, tan dead tissue (devitalized) • Eschar-black/brown necrotic tissue, can be hard or soft Evaluating additional tissue damage: Undermining Separation of tissue from the surface under the edge of the wound • Describe by clock face with patients head at 12 (“undermining is 1 cm from 12 to 4 o’clock”) Tunneling Channel that runs from the wound edge through to other tissue • “tunneling at 9 o’clock, measuring 3 cm long” Wound Drainage and Odor Exudate Fluid from wound • Document the amount, type and odor • Light, moderate, heavy • Drainage can be clear, sanguineous (bloody), serosanguineous (blood-tinged), -

SOCH111 History of Healing Last Modified: 17-Jun-2021
SUBJECT OUTLINE Subject Name: Subject Code: History of Healing SOCH111 SECTION 1 – GENERAL INFORMATION Award/s: Total Course Credit Points: Level: Bachelor of Health Science (Naturopathy) 128 1st Year Bachelor of Health Science (Myotherapy) 96 1st Year Bachelor of Health Science (Nutritional and Dietetic Medicine) 96 1st Year Bachelor of Complementary Medicine 48 1st Year Diploma of Health Science 32 1st Year Duration: 1 Semester Subject is: Core Subject Credit Points: 4 Student Workload: No. timetabled hours per week: No. personal study hours per week: Total hours per week: 6 4 10 Delivery Mode*: ☐ On campus ☒ Online / Digital ☐ Blended ☐ Intensive Weekly Session^ Format/s - 2 sessions per week: ☒ eLearning modules: Lectures: Interactive adaptive online learning modules Tutorials: can include asynchronous tutor moderated discussion forum and activities, learning journal activities or other web-based resources *All modes are supported by the online learning management system which will include subject documents such as handouts, readings and assessment guides. ^A ‘session’ is made up of 3 hours of timetabled / online study time per week unless otherwise specified. Each subject has a set number of sessions as outlined above. Study Pattern: ☒ Full Time ☒ Part Time Pre-requisites: Nil Co-requisites: Nil SECTION 2 – ACADEMIC DETAILS Subject Rationale This subject provides the student with an understanding of the history and philosophy underpinning traditional and other whole medical systems from early human existence to the present day in diverse cultures worldwide. Social, Australian College of Natural Medicine Pty Ltd trading as Endeavour College of Natural Health, FIAFitnation (National CRICOS #00231G, RTO #31489) SOCH111 History of Healing Last modified: 17-Jun-2021 Version: 27.0 Page 1 of 10 cultural and political developments are considered in the evolution of healing and medicine, as well as the parallel developments in anatomy, physiology and other sciences. -

Innate Immunity and Inflammation
ISBTc ‐ Primer on Tumor Immunology and Biological Therapy of Cancer InnateInnate ImmunityImmunity andand InflammationInflammation WillemWillem Overwijk,Overwijk, Ph.D.Ph.D. MDMD AndersonAnderson CancerCancer CenterCenter CenterCenter forfor CancerCancer ImmunologyImmunology ResearchResearch Houston,Houston, TXTX www.allthingsbeautiful.com InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. Adapted from Merriam‐Webster Medical Dictionary • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. • Inflammation: a local response to tissue injury – Rubor (redness) – Calor (heat) – Dolor (pain) – Tumor (swelling) Adapted from Merriam‐Webster Medical Dictionary ““InnateInnate ImmunityImmunity”” andand ““InflammationInflammation”” areare vaguevague termsterms •• SpecificSpecific cellcell typestypes andand moleculesmolecules orchestrateorchestrate specificspecific typestypes ofof inflammationinflammation -

Effect of Exercise Intensity on Cell-Mediated Immunity
sports Perspective Effect of Exercise Intensity on Cell-Mediated Immunity Katsuhiko Suzuki 1,* and Harumi Hayashida 2 1 Faculty of Sport Sciences, Waseda University, 2-579-15 Mikajima, Tokorozawa 359-1192, Japan 2 Faculty of Culture and Sport Policy, Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8503, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-4-2947-6898 Abstract: Moderate-intensity exercise is considered to enhance immune function and to be useful for preventing acute upper respiratory infections and similar conditions. Many people practice low- intensity short-duration exercise with the expectation of a beneficial effect on immunocompetency. However, it is difficult to affirm the existence of definite evidence of such a benefit. In this article, we discuss the effects of low-intensity short-duration exercise on cell-mediated immunity, and contrast them to the effects of high-intensity and long-duration exercise. Whereas high-intensity exercise induces inflammation and reduces cell-mediated immune system function, low-intensity exercise does not appear to have a large effect on either inflammation or cell-mediated immune function. Low-intensity exercises such as walking and yoga, which are helpful to relieve stress, cannot be considered as harmful to the immune system. Although yoga was shown to impose fewer restrictions on breathing and physical strain, the evidence that yoga enhances cell-mediated immunity remains insufficient. Therefore, further studies are needed to examine the exercise mode that may be most effective for improvement of immune functions. Keywords: exercise; walking; yoga; cellular immune system; cytokines; inflammation 1. -

An EANM Procedural Guideline
European Journal of Nuclear Medicine and Molecular Imaging https://doi.org/10.1007/s00259-018-4052-x GUIDELINES Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: an EANM procedural guideline A. Signore1 & F. Jamar2 & O. Israel3 & J. Buscombe4 & J. Martin-Comin5 & E. Lazzeri6 Received: 27 April 2018 /Accepted: 6 May 2018 # The Author(s) 2018 Abstract Introduction Radiolabelled autologous white blood cells (WBC) scintigraphy is being standardized all over the world to ensure high quality, specificity and reproducibility. Similarly, in many European countries radiolabelled anti-granulocyte antibodies (anti-G-mAb) are used instead of WBC with high diagnostic accuracy. The EANM Inflammation & Infection Committee is deeply involved in this process of standardization as a primary goal of the group. Aim The main aim of this guideline is to support and promote good clinical practice despite the complex environment of a national health care system with its ethical, economic and legal aspects that must also be taken into consideration. Method After the standardization of the WBC labelling procedure (already published), a group of experts from the EANM Infection & Inflammation Committee developed and validated these guidelines based on published evidences. Results Here we describe image acquisition protocols, image display procedures and image analyses as well as image interpre- tation criteria for the use of radiolabelled WBC and monoclonal antigranulocyte antibodies. Clinical application for WBC and anti-G-mAb scintigraphy is also described. Conclusions These guidelines should be applied by all nuclear medicine centers in favor of a highly reproducible standardized practice. Keywords Infection . -

Collagen in Wound Healing
bioengineering Review Collagen in Wound Healing Shomita S. Mathew-Steiner, Sashwati Roy and Chandan K. Sen * Indiana Center for Regenerative Medicine and Engineering, School of Medicine, Indiana University, Indianapolis, IN 46202, USA; [email protected] (S.S.M.-S.); [email protected] (S.R.) * Correspondence: [email protected]; Tel.: +1-317-278-2735 Abstract: Normal wound healing progresses through inflammatory, proliferative and remodeling phases in response to tissue injury. Collagen, a key component of the extracellular matrix, plays critical roles in the regulation of the phases of wound healing either in its native, fibrillar conformation or as soluble components in the wound milieu. Impairments in any of these phases stall the wound in a chronic, non-healing state that typically requires some form of intervention to guide the process back to completion. Key factors in the hostile environment of a chronic wound are persistent inflammation, increased destruction of ECM components caused by elevated metalloproteinases and other enzymes and improper activation of soluble mediators of the wound healing process. Collagen, being central in the regulation of several of these processes, has been utilized as an adjunct wound therapy to promote healing. In this work the significance of collagen in different biological processes relevant to wound healing are reviewed and a summary of the current literature on the use of collagen-based products in wound care is provided. Keywords: extracellular matrix; collagen; signaling; inflammation; wound healing; collagen dressings; engineered collagen Citation: Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound 1. Introduction Healing. Bioengineering 2021, 8, 63. Sophisticated regulation by a number of key factors including the environment of the https://doi.org/10.3390/ wound which is rich in extracellular matrix (ECM) drives the process of wound healing [1,2]. -
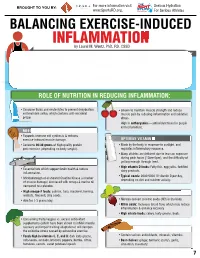
INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M
For more information visit Serious Hydration For more information visit Serious Hydration BROUGHT TO YOU BY: BROUGHT TO YOU BY: For more information visit Serious Hydration www.SportsRD.org. For Serious Athletes www.SportsRD.org. For Serious Athletes www.SportsRD.org. NASA DEVELOPED For Serious Athletes NUTRITIONAL SUPPORT FOR INJURY BALANCING EXERCISE-INDUCED RECOVERY AND RETURN-TO-PLAY INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M. Wentz, PhD, RD, CSSD Vitamin C Citrus fruit, red and green peppers, Antioxidant, wound healing, tissue repair, EXERCISE-INDUCED INFLAMMATION IS THE BODY’S RESPONSE TO INJURY DUE TO INTENSE PHYSICAL ACTIVITY cantaloupe immune function • The immune system response causes redness, swelling, pain. Vitamin A Sweet potato, spinach, carrots, Cell growth and development, • Acute inflammation is a normal response to high-intensity exercise, but prolonged (chronic) tomatoes immune function inflammation is a sustained response that affects the entire body. Vitamin D Sun exposure, oily fish, Promotes calcium absorption and bone health • Prolonged Inflammation: dairy products, fortified foods 1. Causes fatigue, muscle damage and soreness. 2. Limits muscle growth and training progression and increases muscle loss. Calcium Low-fat milk, fortified non-dairy milk, Supports skeletal structure and function 3. Modulating prolonged inflammation may enhance recovery & reduce soreness. low-fat Greek yogurt, cheese, broccoli, kale, fortified orange juice Magnesium Almonds, sesame and sunflower Nucleic acid and protein