Tert-Butanol - Isobutanol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -
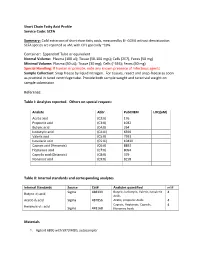
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Supporting Information
Supporting Information Section 1 Components of DCM based coating strippers Table 1. Composition of Klean Strip Premium. CAS # Components Concentration 75-09-2 Dichloromethane 70.0-95.0% 67-56-1 Methanol < 5.0% 127087-87-0 Poly(oxy-1,2-ethandiyl) < 5.0% 124-38-9 Carbon dioxide < 5.0% Table 2. Composition of Klean Strip X. CAS # Components Concentration 75-09-2 Dichloromethane 30.0 – 40.0% 67-56-1 Methanol 15.0 – 26.0% 67-64-1 Acetone < 10.0% 1330-20-7 Xylene < 10.0% 108-88-3 Toluene < 10.0% 100-41-4 Ethylbenzene < 5.0% 64-17-5 Ethyl alcohol < 5.0% 67-63-0 Isopropyl alcohol < 5.0% Section 2 Sample preparation for the dwell time test As Figure S1 shows, a gasket was pasted on the conformal coating surface and a sheet of parafilm attached to the back of the printed circuit board to avoid solvent leakage dur- ing the dwell test. Figure 1. Sample preparation for the dwell time test. Section 3 Thickness measurement of coated PCBs Materials and Equipment Printed circuit boards (PCB), acrylic conformal coating, tape, Dektak stylus profiler (Bruker, Arizona, USA). Methods A piece of tape was attached on a PCB before coating. The coating was applied on the PCB using the same method as dip coating in the dwell time test. The PCB was sta- tioned and dried at room temperature for over 24 hours. The tape was then torn out to create a coating step. The PCB was fixed on the detection table using tapes. The stylus scanned from sub- strate to coated area. -

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -
![[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An](https://docslib.b-cdn.net/cover/9902/agr-biol-chem-vol-36-no-1-p-112-119-1972-studies-of-the-peptide-antibiotic-suzukacillin-part-ii-by-takaaki-ooka-an-399902.webp)
[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An
[Agr. Biol. Chem., Vol. 36, No. 1, p. 112---119,1972] Studies of the Peptide Antibiotic Suzukacillin Part II By Takaaki OOKA and Isao TAKEDA TechnicalResearch Laboratory, Asahi ChemicalIndustry Co., Ltd., Nakadai-cho3-27, Itabashi-kuTokyo ReceivedJuly 26, 1971 Suzukacillin produced by Trichoderma viride 6301 strain was purified and shown to be separated into two components A and B on thin-layer chromatography. The component A was isolated and crystallized from the mixture of components by alumina column chro matography. The component A is composed of six amino acids, Gly, Glu, Ala, Pro, Val, Leu and an unknown amino acid. This unknown amino acid was identified as a-amino isobutyric acid. It is supposed that a-amino isobutyrie acid is biosynthesized mainly from L-valine by the isotopic experiments. Suzukacillin formation by Trichoderma viride 6301 was stimulated by the addition of L-Asn, GABA, L-Ser, Gly and L-Arg into the medium. Trichoderma viride 6301 strain producing Suzu ments were purchased as reagents from Tokyo Kasei kacillin was isolated by the author's laboratory. Co., Ltd. The following radioactive compounds were This organism accumulated noted amount of surplied by Dai-ichi Chemical Co., Ltd., with the in the peptide antibiotic. It was detected on dicated specific activities (ƒÊCi per mmole): Starch- TLC that the peptide antibiotic Suzukacillin (U)14C (30.8), pyruvic acid-(U)-14C (31.5), glycine- consists of two components A and B. A is (U)14C (45.2), L-alanine-(U)-14C (107), L-glutamic acid- (U)-14C (165), L-proline-(U)-14C (165), L-valine-(U)-14C a major and B is a minor component. -

Enhanced Butanol Production by Free and Immobilized Clostridium Sp
Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate Laili Gholizadeh This thesis comprises 30 ECTS credits and is a compulsory part in the Master of Science with a Major in Chemical Engineering – Applied Biotechnology 120 ECTS credits No. 10/2009 Title: Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells using Butyric Acid as Co-Substrate. Author: Laili Gholizadeh Baroghi (e-mail: [email protected]) Master Thesis Subject Category: Biotechnology (Bioprocess Engineering – Biofuels) University College of Borås School of Engineering SE-501 90 BORÅS Telephone: (+46) 033 435 4640 Examiner: Prof. Mohammad Taherzadeh Supervisor and Thesis Advisor: Prof. Shang–Tian Yang Supervisor Address: OSU–Ohio State University 125 Koffolt Laboratories 140 West 19th Ave. Columbus, OH 43210–1185, USA Client: Ohio State University (OSU), Chemical & Biomolecular Engineering Department Prof. Shang–Tian Yang Columbus, Ohio; USA. Date: 08–12–2009 Keywords: Bio-butanol y Acetone–Butanol–Ethanol (ABE) y ABE- fermentation y Butyric acid y Clostridium y C. acetobutylicum ATCC 824 y C. beijerinckii ATCC 55025 y C. beijerinckii BA 101 y C. beijerinckii NCIMB 8052 y Fibrous-bed Bioreactor (FBB) y Batch y Suspended cell culture y Immobilized cell system. DEDICATION I would like to dedicate this M.Sc. Thesis to my beloved Family for all their love and encouragement and for always been supportive of my choices. “I am among those who think that science has great beauty. A scientist in his laboratory is not only a technician: he is also a child placed before natural phenomena, which impress him like a fairy tale.” − Marie Curie ABSTRACT Butanol production by four different Clostridium sp. -

Clinical Trial of the Neuroprotectant Clomethiazole in Coronary Artery Bypass Graft Surgery a Randomized Controlled Trial Robert S
Anesthesiology 2002; 97:585–91 © 2002 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Clinical Trial of the Neuroprotectant Clomethiazole in Coronary Artery Bypass Graft Surgery A Randomized Controlled Trial Robert S. Kong, F.R.C.A.,* John Butterworth, M.D.,† Wynne Aveling, F.R.C.A.,‡ David A. Stump, M.D.,† Michael J. G. Harrison, F.R.C.P.,§ John Hammon, M.D.,ʈ Jan Stygall, M.Sc.,# Kashemi D. Rorie, Ph.D.,** Stanton P. Newman, D.Phil.†† Background: The neuroprotective property of clomethiazole THE efficacy of coronary artery bypass surgery in the has been demonstrated in several animal models of global and relief of angina and in some circumstances in enhancing Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/97/3/585/335909/0000542-200209000-00011.pdf by guest on 29 September 2021 focal brain ischemia. In this study the authors investigated the life expectation is now accepted. It is a tribute to the low effect of clomethiazole on cerebral outcome in patients under- mortality of the procedure that interest is now focused going coronary artery bypass surgery. Methods: Two hundred forty-five patients scheduled for on the neurologic and neuropsychological complica- 1–3 coronary artery bypass surgery were recruited at two centers tions. Adverse neurologic outcomes have been found and prospectively randomized to clomethiazole edisilate to occur in 2–6% of patients and neuropsychological (0.8%), 225 ml (1.8 mg) loading dose followed by a maintenance deficits in 10–30%.4–6 These complications are consid- dose of 100 ml/h (0.8 mg/h) during surgery, or 0.9% NaCl ered to be ischemic in nature, being a consequence of (placebo) in a double-blind trial. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Isobutanol in Marine Gasoline
Isobutanol in Marine Gasoline Glenn Johnston July 11, 2017 Bioeconomy 2017 Washington D.C. 1B: Drivers for Emergence of Biofuels for Maritime Industry © 2012 Gevo, Inc. | 1 Gevo’s Current Business System Gevo Production Facilities Core Near Term Markets Isobutanol Production – Side-by-Side with Ethanol Drop-in Markets - Isobutanol Luverne, MN Isobutanol Specialty Chemicals & Solvents Specialty Gasoline Blendstock (Marine/Off-Road) 15 MGPY EtOH 1.5 MGPY IBA* Isobutanol Hydrocarbon Biorefinery Drop-in Markets - Hydrocarbons South Hydrocarbons Hampton Jet Fuel Resources Silsbee, TX Isooctane (gasoline) © 2016 Gevo, Inc. | 2 Isobutanol Properties Gasoline blending value Gasoline Ethanol Isobutanol RON 95 109 105 MON 85 90 91 Anti-knock Index 90 100 98 RVP (psi) 7-15 19 5.2 Density 20C [kg/m3] 720-775 794 801 Boiling Point (C) 32.2 21.1 26.6 % Heating Value of Gasoline 100 66 84 Oxygen (%w/w) <2.7% 34.7 21.6 isobutanol has low RVP, enabling refiners to blend incremental volumes of butanes and pentanes Marine Research Overview Marine Engine Tested – BRP Envinrude and SeaDoo, Mercury, Volvo-Penta, Yamaha, Tohatsu, Indmar, OMC-Johnson, Honda. Marine Biobutanol over 5 years of research -Alternative Fuel Butanol: Preliminary Investigation on Performance and Emissions of a Marine Two-Stroke Direct Fuel Injection Engine -Impact of Blending Gasoline with lsobutanol Compared to Ethanol on Efficiency, Performance and Emissions of a Recreational Marine 4-Stroke Engine -Gaseous and Particulate Emissions Using Isobutanol-Extended Fuel in Recreational Marine -

Aldehydes and Ketones
Organic Lecture Series Aldehydes And Ketones Chap 16 111 Organic Lecture Series IUPAC names • the parent alkane is the longest chain that contains the carbonyl group • for ketones, change the suffix -e to -one • number the chain to give C=O the smaller number • the IUPAC retains the common names acetone, acetophenone, and benzophenone O O O O Propanone Acetophenone Benzophenone 1-Phenyl-1-pentanone (Acetone) Commit to memory 222 Organic Lecture Series Common Names – for an aldehyde , the common name is derived from the common name of the corresponding carboxylic acid O O O O HCH HCOH CH3 CH CH3 COH Formaldehyde Formic acid Acetaldehyde Acetic acid – for a ketone , name the two alkyl or aryl groups bonded to the carbonyl carbon and add the word ketone O O O Ethyl isopropyl ketone Diethyl ketone Dicyclohexyl ketone 333 Organic Lecture Series Drawing Mechanisms • Use double-barbed arrows to indicate the flow of pairs of e - • Draw the arrow from higher e - density to lower e - density i.e. from the nucleophile to the electrophile • Removing e - density from an atom will create a formal + charge • Adding e - density to an atom will create a formal - charge • Proton transfer is fast (kinetics) and usually reversible 444 Organic Lecture Series Reaction Themes One of the most common reaction themes of a carbonyl group is addition of a nucleophile to form a tetrahedral carbonyl addition compound (intermediate). - R O Nu - + C O Nu C R R R Tetrahedral carbonyl addition compound 555 Reaction Themes Organic Lecture Series A second common theme is -

A Retrospective Comparison of Inappropriate Prescribing Of
Schjøtt and Aßmus BMC Medical Informatics and Decision Making (2019) 19:102 https://doi.org/10.1186/s12911-019-0821-0 RESEARCHARTICLE Open Access A retrospective comparison of inappropriate prescribing of psychotropics in three Norwegian nursing homes in 2000 and 2016 with prescribing quality indicators Jan Schjøtt1,2* and Jörg Aßmus3 Abstract Background: Inappropriate prescribing of psychotropics is a persistent and prevalent problem in nursing homes. The present study compared inappropriate prescribing of psychotropics in nursing homes 16 years apart with prescribing quality indicators. The purpose was to identify any change in inappropriate prescribing of relevance for medical informatics. Methods: Three Norwegian nursing homes were audited in 2000 and 2016 with regard to prescribing quality. Psychotropics among 386 patients in 2000, and 416 patients in 2016, included combinations of antidepressants, antipsychotics, anxiolytics-hypnotics, and antiepileptics. Prescribing quality indicators included psychotropic polypharmacy (defined as concurrent use of three or more psychotropics) and potential inappropriate psychotropic substances or combinations. Furthermore, potential clinically relevant psychotropic interactions were classified as pharmacodynamic or pharmacokinetic using an interaction database. The first ranked (most important) interaction in each patient was selected with the following importance of categories in the database; recommended action > documentation > severity. Three levels (from low to high) within each category were used for ranking. Results: From 2000 to 2016, psychotropic polypharmacy increased from 6.2 to 29.6%, potential inappropriate psychotropic substances was reduced from 17.9 to 11.3% and potential inappropriate psychotropic combinations increased from 7.8 to 27.9%. Changes in polypharmacy and combinations were predominantly associated with prescribing of anxiolytics-hypnotics.