Human Streptococcus Suis Outbreak, Sichuan, China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fantasia Holdings Group Co., Limited 花樣年控股
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Fantasia Holdings Group Co., Limited (Incorporated花樣年控股集團有限公 in the Cayman Islands with limited liability司 ) (Stock Code: 1777) ANNOUNCEMENT OF 2020 ANNUAL RESULTS FINANCIAL HIGHLIGHTS – The Group achieved total contracted sales for the year of 2020 of approximately RMB49.207 billion, representing a year-on-year increase of 35.9%. – The Group’s total revenue was approximately RMB21.759 billion in 2020, representing an increase of 14.0% as compared with RMB19.082 billion in 2019. – The Group’s net profit increased by 16.6% year-on-year to approximately RMB1,751 million. – The profit attributable to the owners of the Company was approximately RMB977 million, representing a year-on-year increase of 11.9%. – Basic earnings per share was RMB16.94 cents. The Board recommended the payment of a final dividend of RMB5.93 cents per share, equivalent to HK$7.05 cents, in cash. 1 The board (the “Board”) of Directors (the “Directors”) of Fantasia Holdings Group Co., Limited (the “Company”) is pleased to announce the audited financial results of the Company and its subsidiaries (the “Group”) for the year ended 31 December 2020 (the “Period”) as follows: CONSOLIDATED STATEMENT OF PROFIT OR -

List of Medical Device Clinical Trial Filing Institutions
List Of Medical Device Clinical Trial Filing Institutions Serial Record number Institution name number Beijing: 5 6 Ge Mechanical temporary 1 agency Beijing Tsinghua Chang Gung Memorial Hospital preparation 201800003 Mechanical temporary 2 agency Plastic Surgery Hospital of Chinese Academy of Medical Sciences preparation 201800008 Mechanical temporary 3 agency Beijing Youan Hospital, Capital Medical University preparation 201800019 Mechanical temporary 4 agency Peking University Shougang Hospital preparation 201800044 Mechanical temporary 5 agency Beijing Cancer Hospital preparation 201800048 Mechanical temporary 6 agency Eye Hospital of China Academy of Chinese Medical Sciences preparation 201800077 Mechanical temporary Beijing Traditional Chinese Medicine Hospital Affiliated to Capital Medical 7 agency University preparation 201800086 Mechanical temporary 8 agency Beijing Anorectal Hospital (Beijing Erlong Road Hospital) preparation 201800103 Mechanical temporary 9 agency Cancer Hospital of Chinese Academy of Medical Sciences preparation 201800108 Serial Record number Institution name number Mechanical temporary Peking Union Medical College Hospital, Chinese Academy of Medical 10 agency Sciences preparation 201800119 Mechanical temporary 11 agency Beijing Luhe Hospital, Capital Medical University preparation 201800128 Mechanical temporary 12 agency Beijing Huilongguan Hospital preparation 201800183 Mechanical temporary 13 agency Beijing Children's Hospital, Capital Medical University preparation 201800192 Mechanical temporary 14 agency -
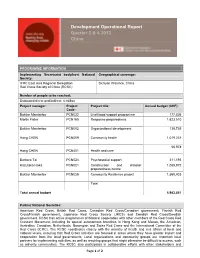
Development Operational Report Quarter 3 & 4 2013 China
Development Operational Report Quarter 3 & 4 2013 China PROGRAMME INFORMATION Implementing Secretariat body/host National Geographical coverage: Society: IFRC East Asia Regional Delegation Sichuan Province, China Red Cross Society of China (RCSC) Number of people to be reached: Estimated direct and indirect: 4 million Project manager: Project Project title: Annual budget (CHF): Code: Baktiar Mambetov PCN022 Livelihood support programme 177,826 Martin Faller PCN165 Response preparedness 1,623,510 Baktiar Mambetov PCN002 Organizational development 139,739 Hong CHEN PCN009 Community health 1,019,231 56,978 Hong CHEN PCN401 Health and care Barbara Tai PCN024 Psychosocial support 311,193 Kazutaka Isaka PCN021 Construction and disaster 1,085,972 preparedness centre Baktiar Mambetov PCN026 Community Resilience project 1,569,403 Total Total annual budget 5,983,851 Partner National Societies: American Red Cross, British Red Cross, Canadian Red Cross/Canadian government, Finnish Red Cross/Finnish government, Japanese Red Cross Society (JRCS) and Swedish Red Cross/Swedish government. RCSC has active programmes of bilateral cooperation with other members of the Red Cross Red Crescent Movement, including its special autonomous branches in Hong Kong and Macao, the American, Australian, Canadian, Netherlands, Norwegian and Swiss Red Cross and the International Committee of the Red Cross (ICRC). The RCSC coordinates closely with the ministry of health and civil affairs at local and national levels, ensuring that Red Cross activities are focused in areas where they have greater impact and cooperation from the local governments. Local organizations and community groups are important local partners for implementing activities, as well as reaching groups that might otherwise be difficult to access, such as minority communities. -

Annual Report 2020 DEFINITIONS (CONTINUED)
CONTENTS I Definitions 2 II Corporate Information 8 III Company Profile 10 IV Chairman’s Statement 12 V Management’s Discussion and Analysis 19 VI Corporate Governance Report 45 VII Report of the Directors 75 VIII Profile of Directors, Supervisors, Senior Management and Employees 99 IX Report of the Supervisory Committee 112 X Independent Auditor's Report 117 XI Consolidated Statement of Profit or Loss and Other Comprehensive Income 123 XII Consolidated Statement of Financial Position 125 XIII Consolidated Statement of Changes in Equity 127 XIV Consolidated Statement of Cash Flows 129 XV Notes to Financial Statements 131 DEFINITIONS In this section, the definitions are presented in alphabetical order (A–Z). I. NAME OF EXPRESSWAY PROJECTS Airport Expressway Chengdu Airport Expressway Chengbei Exit Expressway Chengdu Chengbei Exit Expressway Chengle Expressway Sichuan Chengle (Chengdu-Leshan) Expressway Chengren Expressway Chengdu-Meishan (Renshou) Section of ChengZiLuChi (Chengdu-Zigong-Luzhou-Chishui) Expressway Chengya Expressway Sichuan Chengya (Chengdu-Ya’an) Expressway Chengyu Expressway Chengyu (Chengdu-Chongqing) Expressway (Sichuan Section) Suiguang Expressway Sichuan Suiguang (Suining-Guang’an) Expressway Suixi Expressway Sichuan Suixi (Suining-Xichong) Expressway Tianqiong Expressway Chengdu Tianfu New Area to Qionglai Expressway 2 Sichuan Expressway Company Limited Annual Report 2020 DEFINITIONS (CONTINUED) II. BRANCHES, SUBSIDIARIES AND PRINCIPAL INVESTED COMPANIES Airport Expressway Company Chengdu Airport Expressway Company -

TITLE HERE Introductions to Chengdu Tianfu International Airport and Ziyang City
TITLE HERE Introductions to Chengdu Tianfu International Airport and Ziyang City Chengdu Tianfu International Airport Chengdu Tianfu International Airport locates in Lu Jia Town, jianyang city. Chengdu Tianfu International Airport is only 18 kilometers away from Ziyang CBD while 50 kilometers away from the center of Chengdu CBD. The airport is positioned as Hub Airport, which plans to put into public in 2020. Chengdu Tianfu International Airport has facilitated development of airport economy for Ziyang, which 45-50 km2 has been included into airport economy zone. We plan to develop manufacturing service and high-tech agriculture and form industrial clusters including airport service, modern logistic, cultural innovation, thus making it an international center for foreign exchanges. Our visit this time is expected to exchange ideas with local government and industrial zone which have good practice in this regard, assimilate what works best. Also, we wish to build relationship in airport manufacturing, service sector and agriculture for possible cooperation in future. Ziyang City Located in the central part of Sichuan Basin, Ziyang was established as a prefecture in 1998 and upgraded to the status of city in 2000. It governs Yanjiang District, Anyue County and Lezhi County, covering an area of 5,747 km2 and with a total population of 3.55 million. Ziyang has a long History and rich culture. Back to 35,000 years ago, ancestors of Ziyang people unlocked the gate of the human civilization of Sichuan. In 135BC under the rule of Western Han Dynasty, counties are set up here then followed by state and prefecture, and the history of governmental establishment has stretched to more than 2100 years. -

Study on the Intensive Use of Urban Land—A Case Study of Hill Area in the Middle of Sichuan Province
Available online at www.sciencedirect.com Energy Procedia 5 (2011) 85–89 IACEED2010 Study on the Intensive Use of Urban Land—A Case Study of Hill Area in the Middle of Sichuan Province Wang Youhan Land Resources College of China West Normal University, Nanchong, Sichuan, China, 637009 Abstract Combining with the realities of hill area in the middle of Sichuan province, this paper evaluated the intensive use extent of urban land in 38 counties located in seven cities of hill area in the middle of Sichuan province and provided approaches for intensive utilization of land in the above mentioned area, by analyzing selected indexes and established evaluation index system. © 2011 Published by Elsevier Ltd. Selection and peer-review under responsibility of RIUDS Keywords: intensive utilization of urban land; evaluation system; hill area in the middle of Sichuan province; 1. Introduction Urban land is the foundation of a city’s formation and development, and the efficiency of urban land use is directly related to a city’s vicissitude and regional development. Urban land intensive utilization was explained and defined from different angles and different ranges by many scholars [1]. The evaluation range of the urban land intensive utilization refers to the urban planning and construction land, which is jointly determined by the general planning of city land use and the general planning of city development, including not only the non-agricultural construction area of concentrated continuous area in the main urban zone, but also those areas of basic improvement municipal utilities that scattered in an inner suburban, closely related with cities [2].This article studies the land intensive utilization extent of 38 counties’ in 7 typical cities by using the principal component analysis. -

E2491 Public Disclosure Authorized
E2491 Public Disclosure Authorized Sichuan Small Towns Development Project by WB Loans Summary of EIA Reports (Draft) Public Disclosure Authorized Public Disclosure Authorized China Railway Eryuan Engineering Group Co., Ltd. National Environmental Impact Assessment Certificate Jia No. 3210 May 2010 Public Disclosure Authorized Contents Chapter I General ...........................................................................................................................................1 1.1 Background ................................................................................................................................................. 1 1.1.1 Project origin..................................................................................................................................1 1.1.2 Necessity of construction ...............................................................................................................1 1.2 Basis for EA Report Preparation ................................................................................................................. 1 1.2.1 PRC Laws and Regulations............................................................................................................1 1.2.2 WB Requirements ..........................................................................................................................2 1.2.3 Industry systems of the State..........................................................................................................2 1.2.4 Main technical Guides....................................................................................................................3 -

Fantasia Holdings Group Co., Limited 花樣年控股集團有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 1777)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Fantasia Holdings Group Co., Limited 花樣年控股集團有限公司 (Incorporated in the Cayman Islands with limited liability) (Stock Code: 1777) ANNOUNCEMENT OF UNAUDITED INTERIM RESULTS FOR THE SIX MONTHS ENDED 30 JUNE 2020 FINANCIAL HIGHLIGHTS – The Group’s contracted sales for the period was approximately RMB17,506 million, representing a year-on-year increase of 32.9%. – The Group’s revenue for the period was approximately RMB9,241 million, representing a year-on-year increase of 7.7%. – The Group’s gross profit for the period was approximately RMB3,109 million, representing a year-on-year increase of 22.5%. – The Group’s gross profit margin for the period was approximately 33.6%, representing a year-on-year increase of 4.0 percentage points and maintaining relatively high level in the industry. – The Group’s net profit for the period was approximately RMB277 million, representing a year-on-year increase of 13.4%. Excluding the impact of net exchange loss, the net profit for the period was approximately RMB558 million, representing a year-on-year increase of 97.6%. – Basic earnings per share was RMB1.67 cents (the corresponding period of 2019: RMB1.77 cents). 1 The board (the “Board”) of -

Interim Report Honours and Awards
01777.HK A Leader in Providing Joyful, Colorful and Meaningful Living Space and Experience. Contents Corporate Information Information 02 HonoursHonors and andAwards Awards 03-0403 CorporateSocial Responsibility Social Responsibility 05-0604 ChairmanChairman’s Statement’s Statement 07-0806 Management Discussion Discussion and Analysis and Analysis 09-3012 Disclosure of Interestsof Interests 31-3634 Corporate Governance Governance and Other and Information 37-4039 Other Information ReportIndependent on InterimReview Review of Condensed Repor 4148 Consolidated Financial Statements Condensed Consolidated Consolidated Statement Statement of Comprehensive of Profit Income 4249 or Loss and Other Comprehensive Income Condensed Consolidated Consolidated Statement Statement of Financial ofPosition 43-4450 Financial Position Condensed Consolidated Consolidated Statement Statement of Changes inof Equity Changes 4552 目錄 in Equity Condensed Consolidated Consolidated Statement Statement of Cash Flows of 4654 Cash Flows CONTENTS Notes to to the the Condensed Condensed Consolidated Consolidated Financial Statements Financial 47-4856 Statements Corporate Information Corporate Information DIRECTORS NOMINATION CORPORATE HEAD Executive Directors COMMITTEE OFFICE IN Mr. Pan Jun (Chairman and Mr. Pan Jun HONG KONG Chief Executive Officer) (Committee Chairman) Room 1202–03 Ms. Zeng Jie, Baby Mr. Ho Man New World Tower 1 Mr. Ke Kasheng Ms. Zeng Jie, Baby 16–18 Queen’s Road Central Mr. Zhang Huiming Dr. Liao Jianwen Central Mr. Chen Xinyu Ms. Wong Pui Sze, Priscilla, JP Hong Kong Mr. Guo Shaomu Independent Non-Executive Mr. Huang Ming CORPORATE Directors (resigned on 30 May 2019) HEADQUARTERS IN Mr. Ho Man PEOPLE’S REPUBLIC OF Dr. Liao Jianwen AUDITORS CHINA Ms. Wong Pui Sze, Priscilla, JP Deloitte Touche Tohmatsu Block A, Funian Plaza Mr. -

The Spatial Pattern and Influencing Factors of Rural Tourism in Chengdu-Chongqing Urban Agglomeration Hong HUI , Wei-Yi HE *
2020 2nd International Conference on Arts, Humanity and Economics, Management (AHEM 2020) ISBN: 978-1-60595-685-5 The Spatial Pattern and Influencing Factors of Rural Tourism in Chengdu-Chongqing Urban Agglomeration Hong HUI1,a, Wei-yi HE2,b,* and Ren-jun ZHANG3,c 1,2,3Tourism Management, Chongqing University of Technology, Chongqing, China [email protected], [email protected],[email protected] *Corresponding author Keywords: Chengdu-Chongqing urban agglomeration, Rural tourism, Spatial pattern, Geographic detector, Influencing factors. Abstract. As the fourth national-level urban agglomeration, the Chengdu-Chongqing urban agglomeration has good conditions for the development of tourism industry. It is of great significance to improve the development level of rural tourism in Chengdu-Chongqing region by understanding its spatial pattern and driving factors of rural tourism. This study analyzes the spatial pattern of rural tourism in the Chengdu-Chongqing urban agglomeration and explores its influencing factors by using the geographic detector model. It found that: (1) The spatial distribution of rural tourism in the Chengdu-Chongqing urban agglomeration presents the characteristics of agglomeration of "two big and three small"; (2) The structural ranking of urban agglomeration is the core factor affecting the spatial pattern of rural tourism; (3) Agricultural development level, economic development level, and tourism development level also have important influence on rural tourism; (4) The influence of resource endowments and natural conditions on it is not obvious. Therefore, the development of rural tourism in the Chengdu-Chongqing urban agglomeration should not be limited to the countryside and the resources itself, but should improve the level of urban development, the quality of agriculture and tourism development from the overall perspective of the urban agglomeration. -

Cooperation Intention of Sichuan Companies 2016-Italy
Cooperation Intention of Sichuan Companies 2016-Italy No. Company Name Exhibitors Title Phone Email Website Address Exhibit/Product Cooperation Intention Company Profile Export Country Industry 起动机、发电机及各 寻找潜在客户,从事客户委托的现代汽车和通 周志文 董事长 scjidao@hotmail 美国、欧洲、加拿 1 四川极道电装实业有限公司 www.scjddz.com 成都市郫县现代工业港北区港泰大道300号 类控制阀(TVSV. 用动力起动机、发电机及各类控制阀等产品的 汽车零配件 .com 大、日本 高琳 外贸 13880201699 EGR阀等 研究、开发、生产、出口和OEM配套 Starter, generator and Look for potential clients; study, develop, produce, We mainly focuses on the research, development, production and Zhou Zhiwen Board Chairman No.300 GangTai Ave, North District of Sichuan Jidao Electrical Equipment Industrial scjidao@hotmail all kinds of control export products like power starter, generator and sales of starters and alternators in automobiles and general US, Europ, Canada, www.scjddz.com Chengdu Modern Industry Port, Pi County, Automotive part Co., Ltd .com valve like TVSV and various of control valve of Hyundai Motor and engines, with voltage from 12V to 24V, and power from 0.4kw to Japan Chengdu, Sichuan, China Gao Lin Overseas Sales 13880201699 EGR valve General Motor and OEM 9kw. 吴敏 经理 13881741131 [email protected] 汽车蓄电池检测仪、 2 成都蓝格尔科技有限公司 www.lancol.com 成都现代工业港北片区港大路 寻求合作 汽车零配件 m 各类汽车检测仪器 杨韵爽 经理 15982327803 Lancol is a high-tech enterprise located in Chengdu of western China, focused on the development of innovative technologies for battery management solutions. Since pioneering a new standard Wu Min Manager 13881741131 in battery testers with conductance technology, Lancol was established on basis of higher standard of the industry requirement with clear business objective: to become the industry Battery analyzer, all [email protected] Gangda Avenue,North Section,Modern leader status. -

Sichuan Ziyang Green Transformation and Development Project
Resettlement Plan (Draft) Project number: 51189-001 April 2018 People’s Republic of China: Sichuan Ziyang Green Transformation and Development Project Prepared by the Project Management Office of the Ziyang Municipal Government for the Asian Development Bank CURRENCY EQUIVALENTS (as of 19 April 2018) Currency unit – yuan (CNY) CNY1.00 = $0.1594 $1.00 = CNY 6.2744 ABBREVIATIONS ADB – Asian Development Bank EIA – environmental impact assessment EMP – environmental management plan GRM – grievance redress mechanism ha – hectare LURT – land use rights transfer mu – a Chinese unit of measurement (1 mu = 0.067 hectares) O&M – operation and maintenance PAM – project administration manual PMO – project management office PRC – People’s Republic of China R&D – research and development SZHTDZ – Sichuan Ziyang High Technology Development Zone TA – technical assistance TVET – technical and vocational education and training YREB – Yangtze River Economic Belt ZMG – Ziyang Municipal Government NOTE In this report, "$" refers to United States dollars. This resettlement plan is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments