18.357 Interfacial Phenomena, Lectures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Life of a Surface Bubble
molecules Review The Life of a Surface Bubble Jonas Miguet 1,†, Florence Rouyer 2,† and Emmanuelle Rio 3,*,† 1 TIPS C.P.165/67, Université Libre de Bruxelles, Av. F. Roosevelt 50, 1050 Brussels, Belgium; [email protected] 2 Laboratoire Navier, Université Gustave Eiffel, Ecole des Ponts, CNRS, 77454 Marne-la-Vallée, France; fl[email protected] 3 Laboratoire de Physique des Solides, CNRS, Université Paris-Saclay, 91405 Orsay, France * Correspondence: [email protected]; Tel.: +33-1691-569-60 † These authors contributed equally to this work. Abstract: Surface bubbles are present in many industrial processes and in nature, as well as in carbon- ated beverages. They have motivated many theoretical, numerical and experimental works. This paper presents the current knowledge on the physics of surface bubbles lifetime and shows the diversity of mechanisms at play that depend on the properties of the bath, the interfaces and the ambient air. In particular, we explore the role of drainage and evaporation on film thinning. We highlight the existence of two different scenarios depending on whether the cap film ruptures at large or small thickness compared to the thickness at which van der Waals interaction come in to play. Keywords: bubble; film; drainage; evaporation; lifetime 1. Introduction Bubbles have attracted much attention in the past for several reasons. First, their ephemeral Citation: Miguet, J.; Rouyer, F.; nature commonly awakes children’s interest and amusement. Their visual appeal has raised Rio, E. The Life of a Surface Bubble. interest in painting [1], in graphism [2] or in living art. -

On the Shape of Giant Soap Bubbles
On the shape of giant soap bubbles Caroline Cohena,1, Baptiste Darbois Texiera,1, Etienne Reyssatb,2, Jacco H. Snoeijerc,d,e, David Quer´ e´ b, and Christophe Claneta aLaboratoire d’Hydrodynamique de l’X, UMR 7646 CNRS, Ecole´ Polytechnique, 91128 Palaiseau Cedex, France; bLaboratoire de Physique et Mecanique´ des Milieux Het´ erog´ enes` (PMMH), UMR 7636 du CNRS, ESPCI Paris/Paris Sciences et Lettres (PSL) Research University/Sorbonne Universites/Universit´ e´ Paris Diderot, 75005 Paris, France; cPhysics of Fluids Group, University of Twente, 7500 AE Enschede, The Netherlands; dMESA+ Institute for Nanotechnology, University of Twente, 7500 AE Enschede, The Netherlands; and eDepartment of Applied Physics, Eindhoven University of Technology, 5600 MB, Eindhoven, The Netherlands Edited by David A. Weitz, Harvard University, Cambridge, MA, and approved January 19, 2017 (received for review October 14, 2016) We study the effect of gravity on giant soap bubbles and show The experimental setup dedicated to the study of such large 2 that it becomes dominant above the critical size ` = a =e0, where bubbles is presented in Experimental Setup, before information p e0 is the mean thickness of the soap film and a = γb/ρg is the on Experimental Results and Model. The discussion on the asymp- capillary length (γb stands for vapor–liquid surface tension, and ρ totic shape and the analogy with inflated structures is presented stands for the liquid density). We first show experimentally that in Analogy with Inflatable Structures. large soap bubbles do not retain a spherical shape but flatten when increasing their size. A theoretical model is then developed Experimental Setup to account for this effect, predicting the shape based on mechan- The soap solution is prepared by mixing two volumes of Dreft© ical equilibrium. -

Soap Films: Statics and Dynamics
Soap Films: Statics and Dynamics Frederik Brasz January 8, 2010 1 Introduction Soap films and bubbles have long fascinated humans with their beauty. The study of soap films is believed to have started around the time of Leonardo da Vinci. Since then, research has proceeded in two distinct directions. On the one hand were the mathematicians, who were concerned with finding the shapes of these surfaces by minimizing their area, given some boundary. On the other hand, physical scientists were trying to understand the properties of soap films and bubbles, from the macroscopic behavior down to the molecular description. A crude way to categorize these two approaches is to call the mathematical shape- finding approach \statics" and the physical approach \dynamics", although there are certainly problems involving static soap films that require a physical description. This is my implication in this paper when I divide the discussion into statics and dynamics. In the statics section, the thickness of the film, concentration of surfactants, and gravity will all be quickly neglected, as the equilibrium shape of the surface becomes the only matter of importance. I will explain why for a soap film enclosing no volume, the mean curvature must everywhere be equal to zero, and why this is equivalent to the area of the film being minimized. For future reference, a minimal surface is defined as a two-dimensional surface with mean curvature equal to zero, so in general soap films take the shape of minimal surfaces. Carrying out the area minimization of a film using the Euler-Lagrange equation will result in a nonlinear partial differential equation known as Lagrange's Equation, which all minimal surfaces must satisfy. -
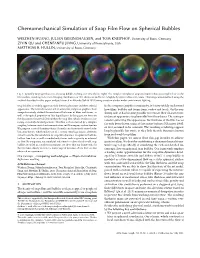
Chemomechanical Simulation of Soap Film Flow on Spherical Bubbles
Chemomechanical Simulation of Soap Film Flow on Spherical Bubbles WEIZHEN HUANG, JULIAN ISERINGHAUSEN, and TOM KNEIPHOF, University of Bonn, Germany ZIYIN QU and CHENFANFU JIANG, University of Pennsylvania, USA MATTHIAS B. HULLIN, University of Bonn, Germany Fig. 1. Spatially varying iridescence of a soap bubble evolving over time (left to right). The complex interplay of soap and water induces a complex flowonthe film surface, resulting in an ever changing distribution of film thickness and hence a highly dynamic iridescent texture. This image was simulated using the method described in this paper, and path-traced in Mitsuba [Jakob 2010] using a custom shader under environment lighting. Soap bubbles are widely appreciated for their fragile nature and their colorful In the computer graphics community, it is now widely understood appearance. The natural sciences and, in extension, computer graphics, have how films, bubbles and foams form, evolve and break. On the ren- comprehensively studied the mechanical behavior of films and foams, as dering side, it has become possible to recreate their characteristic well as the optical properties of thin liquid layers. In this paper, we focus on iridescent appearance in physically based renderers. The main pa- the dynamics of material flow within the soap film, which results in fasci- rameter governing this appearance, the thickness of the film, has so nating, extremely detailed patterns. This flow is characterized by a complex far only been driven using ad-hoc noise textures [Glassner 2000], coupling between surfactant concentration and Marangoni surface tension. We propose a novel chemomechanical simulation framework rooted in lu- or was assumed to be constant. -

THE GEOMETRY of BUBBLES and FOAMS University of Illinois Department of Mathematics Urbana, IL, USA 61801-2975 Abstract. We Consi
THE GEOMETRY OF BUBBLES AND FOAMS JOHN M. SULLIVAN University of Illinois Department of Mathematics Urbana, IL, USA 61801-2975 Abstract. We consider mathematical models of bubbles, foams and froths, as collections of surfaces which minimize area under volume constraints. The resulting surfaces have constant mean curvature and an invariant notion of equilibrium forces. The possible singularities are described by Plateau's rules; this means that combinatorially a foam is dual to some triangulation of space. We examine certain restrictions on the combina- torics of triangulations and some useful ways to construct triangulations. Finally, we examine particular structures, like the family of tetrahedrally close-packed structures. These include the one used by Weaire and Phelan in their counterexample to the Kelvin conjecture, and they all seem useful for generating good equal-volume foams. 1. Introduction This survey records, and expands on, the material presented in the author's series of two lectures on \The Mathematics of Soap Films" at the NATO School on Foams (Cargese, May 1997). The first lecture discussed the dif- ferential geometry of constant mean curvature surfaces, while the second covered the combinatorics of foams. Soap films, bubble clusters, and foams and froths can be modeled math- ematically as collections of surfaces which minimize their surface area sub- ject to volume constraints. Each surface in such a solution has constant mean curvature, and is thus called a cmc surface. In Section 2 we examine a more general class of variational problems for surfaces, and then concen- trate on cmc surfaces. Section 3 describes the balancing of forces within a cmc surface. -

The Dynamics of a Viscous Soap Film with Soluble Surfactant Jean-Marc Chomaz
The dynamics of a viscous soap film with soluble surfactant Jean-Marc Chomaz To cite this version: Jean-Marc Chomaz. The dynamics of a viscous soap film with soluble surfactant. Jour- nal of Fluid Mechanics, Cambridge University Press (CUP), 2001, 442 (september), pp.387-409. 10.1017/s0022112001005213. hal-01025351 HAL Id: hal-01025351 https://hal-polytechnique.archives-ouvertes.fr/hal-01025351 Submitted on 11 Sep 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J. Fluid Mech. (2001), vol. 442, pp. 387–409. Printed in the United Kingdom 387 c 2001 Cambridge University Press The dynamics of a viscous soap film with soluble surfactant By JEAN-MARC CHOMAZ LadHyX, CNRS–Ecole Polytechnique, 91128 Palaiseau, France (Received 25 October 1999 and in revised form 24 March 2001) Nearly two decades ago, Couder (1981) and Gharib & Derango (1989) used soap films to perform classical hydrodynamics experiments on two-dimensional flows. Recently soap films have received renewed interest and experimental investigations published in the past few years call for a proper analysis of soap film dynamics. In the present paper, we derive the leading-order approximation for the dynamics of a flat soap film under the sole assumption that the typical length scale of the flow parallel to the film surface is large compared to the film thickness. -

FOAM • Boulder School for Condensed Matter and Materials Physics July 1-26, 2002: Physics of Soft Condensed Matter
THE PHYSICS OF FOAM • Boulder School for Condensed Matter and Materials Physics July 1-26, 2002: Physics of Soft Condensed Matter Douglas J. DURIAN 1. Introduction UCLA Physics & Formation Astronomy Los Angeles, CA 90095- Microscopics 1547 2. Structure <[email protected]> Experiment Simulation 3. Stability Coarsening Drainage 4. Rheology Linear response Rearrangement & flow Foam is… • …a random packing of bubbles in a relatively small amount of liquid containing surface-active impurities – Four levels of structure: – Three means of time evolution: • Gravitational drainage • Film rupture • Coarsening (gas diffusion from smaller to larger bubbles) Foam is… • …a most unusual form of condensed matter –Like a gas: • volume ~ temperature / pressure – Like a liquid: • Flow without breaking • Fill any shape vessel – Under large force, bubbles rearrange their packing configuration – Like a solid: • Support small shear forces elastically – Under small force, bubbles distort but don’t rearrange Foam is… • Everyday life: – detergents – foods (ice cream, meringue, beer, cappuccino, ...) – cosmetics (shampoo, mousse, shaving cream, tooth paste, ...) • Unique applications: – firefighting – isolating toxic materials – physical and chemical separations – oil recovery – cellular solids •• ffamiliar!amiliar! • Undesirable occurrences: – mechanical agitation of multicomponent liquid •i•immppoorrttaanntt!! – pulp and paper industry – paint and coating industry – textile industry – leather industry – adhesives industry – polymer industry – food processing -

A Model for Soap Film Dynamics with Evolving Thickness
A Model for Soap Film Dynamics with Evolving Thickness SADASHIGE ISHIDA*, PETER SYNAK*, IST Austria FUMIYA NARITA, Unaffiliated TOSHIYA HACHISUKA, The University of Tokyo CHRIS WOJTAN, IST Austria Fig. 1. Our model simulates the evolution of soap films, leading to detailed advection patterns (left) and interplays between draining, evaporation, capillary waves, and ruptures in a foam (right). Previous research on animations of soap bubbles, films, and foams largely ACM Reference Format: focuses on the motion and geometric shape of the bubble surface. These Sadashige Ishida*, Peter Synak*, Fumiya Narita, Toshiya Hachisuka, and Chris works neglect the evolution of the bubble’s thickness, which is normally re- Wojtan. 2020. A Model for Soap Film Dynamics with Evolving Thickness. sponsible for visual phenomena like surface vortices, Newton’s interference ACM Trans. Graph. 39, 4, Article 31 (July 2020), 11 pages. https://doi.org/10. patterns, capillary waves, and deformation-dependent rupturing of films in 1145/3386569.3392405 a foam. In this paper, we model these natural phenomena by introducing the film thickness as a reduced degree of freedom in the Navier-Stokes equations 1 INTRODUCTION and deriving their equations of motion. We discretize the equations on a non- This paper concerns the animation of soap films, bubbles, and foams. manifold triangle mesh surface and couple it to an existing bubble solver. In These natural phenomena exhibit fascinating and beautiful com- doing so, we also introduce an incompressible fluid solver for 2.5D films and a novel advection algorithm for convecting fields across non-manifold sur- plexity in their geometry, dynamics, and color. -

The World of Soap Bubbles: from Fantasy to Science
The World of Soap Bubbles: From Fantasy to Science Pallavi Ghalsasi1* School of Science and Engineering, Navrachana University, Vasna-Bhayli Road, Vadodara- 391 410, Gujarat, India Email: [email protected], [email protected] Abstract: Soap bubbles have been one of the most fascinating play activities for almost every child. The various ways of making different size bubbles, their reproducible geometrical forms, their beautiful colours, and their tenacity, the emotional involvement in their sustainability before they perish and the way they fly, have always added to the cascaded excitement in the play with soap bubbles. It is not limited to just children but also has been of profound interest to poets, scientists, mathematicians, artists and architects for hundreds of years. Apart from the fantasy driving most of the innocent playful and dreamy minds, soap bubbles also display a plethora of deep science and a variety of forces shaping nature. Present article depicts a way to appreciate science through the lenses of multidisciplinary dimensions, the same manner in which the very Nature has unfolded it to us. The article then reviews enormous progress the human beings with curious minds have made to search for the origin of the observations pertaining to soap bubbles, either made casually or consciously. Although the article is based on various aspects of science of soap bubbles including some demonstrations, the approach can be extended to many topics in all the disciplines. Keywords: Soap bubbles, soft matter, interference, fluid mechanics, structures Introduction: For a very long time, various artists and poets have illustrated their paintings or poems themed around soap bubbles in Europe and America dated back in 16th century. -

The Mathematics of Soap Films
Mathematics and Soap Films John Oprea Cleveland State University Surface tension creates a “skin” on a liquid whose molecules are polar. Example: H2O is polar, so water has a “skin”. Soap reduces surface tension by adding long polar molecules with hydrocarbon tails. Surface tension dominance, but with noticeable effects of gravity Surface tension pulls a soap film as tight as it can be. 1st Principle of Soap Films. A soap film minimizes its surface area. But what is the exact geometry dictated by the force due to surface tension? To see this, let’s analyze a piece of a soap film surface that is expanded outward by an applied pressure p. Take a piece of the film given by two perpendicular (tangent) directions and compute the work done to expand the surface area under some pressure. Also, if we take p = pressure and S = surface area, then The change in surface area is given by A Physicist’s first words: “Neglect the higher order Term!” Laplace-Young Equation Laplace-Young involves Mean Curvature! Definition. A surface S is minimal if H = 0. Theorem. A soap film is a minimal surface. Questions What property does a soap bubble have? What happens when bubbles fuse? Does the Laplace-Young equation have medical consequences? Alveoli are modeled Alveoli by spheres which expand when we inhale and contract when we exhale. When is the pressure difference the greatest? So how can we ever inhale? It was the development of artificial surfactant in the 1960’s that was essential to the survival of premature babies! Now consider the “loop on a hoop” experiment. -

On the Thickness of Soap Films: an Alternative to Frankel's
J. Fluid Mech. (2008), vol. 602, pp. 119–127. c 2008 Cambridge University Press 119 doi:10.1017/S0022112008000955 Printed in the United Kingdom On the thickness of soap films: an alternative to Frankel’s law ERNST A. VAN NIEROP, BENOIT SCHEID AND HOWARD A. STONE School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA (Received 17 December 2007 and in revised form 13 February 2008) The formation of soap films by vertical withdrawal from a bath is typically described by Frankel’s law, which assumes rigid film ‘walls’ and shear-like dynamics. Since most soap films have interfaces that are not rigid, and as the flow in the withdrawal of thin free films is typically extensional, we reconsider the theory of soap film formation. By assuming extensional flow dominated by surface viscous stresses we find that the film thickness scales as the two-thirds power of the withdrawal speed U. This speed dependence is also predicted by Frankel’s law; the difference lies in the origin of the viscous resistance which sets the pre-factor. When bulk viscous stresses are important the speed dependence can vary between U 2/3 and U 2. 1. Introduction It is simple to make a soap film by withdrawing a wire frame from a bath of soapy water as sketched in figure 1(a). Whether demonstrating colourful interference patterns in a soap film or determining properties of an industrial foam, an accurate prediction of the soap film thickness is important. Frankel is credited with showing that if the soap film interfaces are assumed to be rigid and inextensible, then for withdrawal the film thickness h0 varies as (Mysels, Shinoda & Frankel 1959) 2/3 h0 ηU 2/3 =1.89 =1.89 Ca , (1.1) c γ √ where c = γ/ρg is the capillary length, η is the viscosity of the liquid, U is the speed at which the film is withdrawn from the bath, γ is the surface tension and Ca is the capillary number. -

Foams and Honeycombs
A reprint from American Scientist the magazine of Sigma Xi, The Scientific Research Society This reprint is provided for personal and noncommercial use. For any other use, please send a request to Permissions, American Scientist, P.O. Box 13975, Research Triangle Park, NC, 27709, U.S.A., or by electronic mail to [email protected]. ©Sigma Xi, The Scientific Research Society and other rightsholders Foams and Honeycombs For centuries, the precise architecture of soap foams has been a source of wonder to children and a challenge to mathematicians Erica G. Klarreich early all of us have been enchanted ble foam, one in which all the bubbles in the sciences that aesthetically pleas- Nat some time in our lives by the have the same volume. Kelvin realized ing solutions seem to have a higher fragile symmetry of soap bubbles and that this question could be boiled down chance of being correct than more com- soap films. But few have had the privi- to the following purely mathematical plicated, less symmetric ones (a topic lege of being introduced to them by a problem: What is the best way to divide elaborated in these pages by James W. great scientist, as Agnes Gardner King three-dimensional space into cells of McAllister in March–April 1998). As did. In the fall of 1887, the fledgling equal volume, if one wants to minimize Oxford University mathematician and painter paid a visit to her uncle, Lord the surface area of the cell walls? physicist Roger Penrose put it, Kelvin (then Sir William Thomson), a Over the course of two months, I have noticed on many occasions in physicist who was already renowned Kelvin searched for the shape that my own work where there might, for his work in thermodynamics.