The Chemistry of Soap Bubbles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Governing Equations for Simulations of Soap Bubbles
University of California Merced Capstone Project Governing Equations For Simulations Of Soap Bubbles Author: Research Advisor: Ivan Navarro Fran¸cois Blanchette 1 Introduction The capstone project is an extension of work previously done by Fran¸coisBlanchette and Terry P. Bigioni in which they studied the coalescence of drops with either a hori- zontal reservoir or a drop of a different size (Blanchette and Bigioni, 2006). Specifically, they looked at the partial coalescence of a drop, which leaves behind a smaller "daugh- ter" droplet due to the incomplete merging process. Numerical simulations were used to study coalescence of a drop slowly coming into contact with a horizontal resevoir in which the fluid in the drop is the same fluid as that below the interface (Blanchette and Bigioni, 2006). The research conducted by Blanchette and Bigioni starts with a drop at rest on a flat interface. A drop of water will then merge with an underlying resevoir (water in this case), forming a single interface. Our research involves using that same numerical ap- proach only this time a soap bubble will be our fluid of interest. Soap bubbles differ from water drops on the fact that rather than having just a single interface, we now have two interfaces to take into account; the air inside the soap bubble along with the soap film on the boundary, and the soap film with any other fluid on the outside. Due to this double interface, some modifications will now be imposed on the boundary conditions involving surface tension along the interface. Also unlike drops, soap bubble thickness is finite which will mean we must keep track of it. -

The Life of a Surface Bubble
molecules Review The Life of a Surface Bubble Jonas Miguet 1,†, Florence Rouyer 2,† and Emmanuelle Rio 3,*,† 1 TIPS C.P.165/67, Université Libre de Bruxelles, Av. F. Roosevelt 50, 1050 Brussels, Belgium; [email protected] 2 Laboratoire Navier, Université Gustave Eiffel, Ecole des Ponts, CNRS, 77454 Marne-la-Vallée, France; fl[email protected] 3 Laboratoire de Physique des Solides, CNRS, Université Paris-Saclay, 91405 Orsay, France * Correspondence: [email protected]; Tel.: +33-1691-569-60 † These authors contributed equally to this work. Abstract: Surface bubbles are present in many industrial processes and in nature, as well as in carbon- ated beverages. They have motivated many theoretical, numerical and experimental works. This paper presents the current knowledge on the physics of surface bubbles lifetime and shows the diversity of mechanisms at play that depend on the properties of the bath, the interfaces and the ambient air. In particular, we explore the role of drainage and evaporation on film thinning. We highlight the existence of two different scenarios depending on whether the cap film ruptures at large or small thickness compared to the thickness at which van der Waals interaction come in to play. Keywords: bubble; film; drainage; evaporation; lifetime 1. Introduction Bubbles have attracted much attention in the past for several reasons. First, their ephemeral Citation: Miguet, J.; Rouyer, F.; nature commonly awakes children’s interest and amusement. Their visual appeal has raised Rio, E. The Life of a Surface Bubble. interest in painting [1], in graphism [2] or in living art. -

Weighted Transparency: Literal and Phenomenal
Frei Otto soap bubble model (top left), Antonio Gaudi gravity model (bottom left), Miguel Fisac fabric form concrete wall (right) Weighted Transparency: Literal and Phenomenal SPRING 2020, OPTION STUDIO VISITING ASSOCIATE PROFESSOR NAOMI FRANGOS “As soon as we adventure on the paths of the physicist, we learn to weigh and to measure, to deal with time and space and mass and their related concepts, and to find more and more our knowledge expressed and our needs satisfied through the concept of number, as in the dreams and visions of Plato and Pythagoras; for modern chemistry would have gladdened the hearts of those great philosophic dreamers.” On Growth and Form, D’Arcy Wentworth Thompson Today’s maker architect is such a dreamt physicist. What is at stake is the ability to balance critical form-finding informed by the behavior of matter itself with the rigor of precision afforded by computational thinking. This studio transposes ideas of cross- disciplinarity in design, art, science, engineering and material studies pioneered by our forerunner master-builders into built prototypes by studying varying degrees of literal and phenomenal transparency achieved through notions of weight. Working primarily with a combination of plaster/concrete and glass/porcelain, juxtapositions are provoked between solid and void, heavy and light, opaque and transparent, smooth and textured, volume and surface. How can the actual weight of a material affect its sense of mass? How can mass capture the phenomena of weightlessness? How can physical material properties dictate appearances beyond optical transparency? Using dynamic matter/flexible formwork (i.e. salt, sand, gravel, fabric), suspended/submerged and compression/ expansion systems, weight plays a major role in deriving methods of fabrication and determining experiential qualities of made artifacts. -

Vibrations of Fractal Drums
VOLUME 67, NUMBER 21 PHYSICAL REVIEW LETTERS 18 NOVEMBER 1991 Vibrations of Fractal Drums B. Sapoval, Th. Gobron, and A. Margolina "' Laboratoire de Physique de la Matiere Condensee, Ecole Polytechnique, 91128 Palaiseau CEDEX, France (Received l7 July l991) Fractal boundary conditions drastically alter wave excitations. The low-frequency vibrations of a membrane bounded by a rigid fractal contour are observed and localized modes are found. The first lower eigenmodes are computed using an analogy between the wave and the diffusion equations. The fractal frontier induces a strong confinement of the wave analogous to superlocalization. The wave forms exhibit singular derivatives near the boundary. PACS numbers: 64.60.Ak, 03.40.Kf, 63.50.+x, 71.55.3v Objects with irregular geometry are ubiquitous in na- shown in Fig. 2. ture and their vibrational properties are of general in- The observation of confined modes was a surprise be- terest. For instance, the dependence of sea waves on the cause, in principle, the symmetry of the structure forbids topography of coastlines is a largely unanswered question. their existence. In fact, this experimental localization is The emergence of fractal geometry was a significant due both to damping and to the existence of narrow paths breakthrough in the description of irregularity [11. It has in the geometry of the membrane. If only one of the been suggested that the very existence of some of the equivalent regions like A is excited, then a certain time T, fractal structures found in nature is attributable to a called the delocalization time is necessary for the excita- self-stabilization due to their ability to damp harmonic tion to travel from A to B. -

Memoirs of a Bubble Blower
Memoirs of a Bubble Blower BY BERNARD ZUBROVVSKI Reprinted from Technology Review, Volume 85, Number 8, Nov/Dec 1982 Copyright 1982, Alumni Association of the Massachusetts Institute of Technolgy, Cambridge, Massachusetts 02139 EDUCATION A SPECIAL REPORT Memoirs N teaching science to children, I have dren go a step further by creating three- found that the best topics are those dimensional clusters of bubbles that rise I that are equally fascinating to chil• up a plexiglass tube. These bubbles are dren and adults alike. And learning is not as regular as those in the honeycomb most enjoyable when teacher and students formation, but closer observation reveals are exploring together. Blowing bubbles that they have a definite pattern: four provides just such qualities. bubbles (and never more than four) are Bubble blowing is an exciting and fruit• usually in direct contact with one another, ful way for children to develop basic in• with their surfaces meeting at a vertex at tuition in science and mathematics. And angles of about 109 degrees. And each working with bubbles has prompted me to of a bubble in the cluster will have roughly the wander into various scientific realms such same number of sides, a fact that turns out as surface physics, cellular biology, topol• to be scientifically significant. ogy, and architecture. Bubbles and soap Bubble In the 1940s, E.B. Matzke, a well- films are also aesthetically appealing, and known botanist who experimented with the children and I have discovered bubbles bubbles, discovered that bubbles in an to be ideal for making sculptures. In fact, array have an average of 14 sides. -

Computer Graphics and Soap Bubbles
Computer Graphics and Soap Bubbles Cheung Leung Fu & Ng Tze Beng Department of Mathematics National University of Singapore Singapore 0511 Soap Bubbles From years of study and of contemplation A boy, with bowl and straw, sits and blows, An old man brews a work of clarity, Filling with breath the bubbles from the bowl. A gay and involuted dissertation Each praises like a hymn, and each one glows; Discoursing on sweet wisdom playfully Into the filmy beads he blows his soul. An eager student bent on storming heights Old man, student, boy, all these three Has delved in archives and in libraries, Out of the Maya.. foam of the universe But adds the touch of genius when he writes Create illusions. None is better or worse A first book full of deepest subtleties. But in each of them the Light of Eternity Sees its reflection, and burns more joyfully. The Glass Bead Game Hermann Hesse The soap bubble, a wonderful toy for children, a motif for poetry, is at the same time a mathematical entity that has a fairly long history. Thanks to the advancement of computer graphics which led to solutions of decade-old conjectures, 'soap bubbles' have become once again a focus of much mathematical research in recent years. Anyone who has seen or played with a soap bubble knows that it is a spherical object. It is indeed a sphere if one defines a soap bubble to be a surface minimizing the area among all surfaces enclosing the same given volume. Relaxing this condition, mathematicians defined a soap bubble or surface of constant mean curvature to be a surface which is locally the stationary point of the area function when compared again locally to neighbouring surfaces enclosing the same volume. -

Graphs in Nature
Graphs in Nature David Eppstein University of California, Irvine Algorithms and Data Structures Symposium (WADS) August 2019 Inspiration: Steinitz's theorem Purely combinatorial characterization of geometric objects: Graphs of convex polyhedra are exactly the 3-vertex-connected planar graphs Image: Kluka [2006] Overview Cracked surfaces, bubble foams, and crumpled paper also form natural graph-like structures What properties do these graphs have? How can we recognize and synthesize them? I. Cracks and Needles Motorcycle graphs: Canonical quad mesh partitioning Problem: partition irregular quad-mesh into regular submeshes [Eppstein et al. 2008] Inspiration: Light cycle game from TRON movies Mesh partitioning method Grow cut paths outwards from each irregular (non-degree-4) vertex Cut paths continue straight across regular (degree-4) vertices They stop when they run into another path Result: approximation to optimal partition (exact optimum is NP-complete) Mesh-free motorcycle graphs Earlier... Motorcycles move from initial points with given velocities When they hit trails of other motorcycles, they crash [Eppstein and Erickson 1999] Application of mesh-free motorcycle graphs Initially: A simplified model of the inward movement of reflex vertices in straight skeletons, a rectilinear variant of medial axes with applications including building roof construction, folding and cutting problems, surface interpolation, geographic analysis, and mesh construction Later: Subroutine for constructing straight skeletons of simple polygons [Cheng and Vigneron 2007; Huber and Held 2012] Image: Huber [2012] Construction of mesh-free motorcycle graphs Main ideas: Define asymmetric distance: Time when one motorcycle would crash into another's trail Repeatedly find closest pair and eliminate crashed motorcycle Image: Dancede [2011] O(n17=11+) [Eppstein and Erickson 1999] Improved to O(n4=3+) [Vigneron and Yan 2014] Additional log speedup using mutual nearest neighbors instead of closest pairs [Mamano et al. -

On the Shape of Giant Soap Bubbles
On the shape of giant soap bubbles Caroline Cohena,1, Baptiste Darbois Texiera,1, Etienne Reyssatb,2, Jacco H. Snoeijerc,d,e, David Quer´ e´ b, and Christophe Claneta aLaboratoire d’Hydrodynamique de l’X, UMR 7646 CNRS, Ecole´ Polytechnique, 91128 Palaiseau Cedex, France; bLaboratoire de Physique et Mecanique´ des Milieux Het´ erog´ enes` (PMMH), UMR 7636 du CNRS, ESPCI Paris/Paris Sciences et Lettres (PSL) Research University/Sorbonne Universites/Universit´ e´ Paris Diderot, 75005 Paris, France; cPhysics of Fluids Group, University of Twente, 7500 AE Enschede, The Netherlands; dMESA+ Institute for Nanotechnology, University of Twente, 7500 AE Enschede, The Netherlands; and eDepartment of Applied Physics, Eindhoven University of Technology, 5600 MB, Eindhoven, The Netherlands Edited by David A. Weitz, Harvard University, Cambridge, MA, and approved January 19, 2017 (received for review October 14, 2016) We study the effect of gravity on giant soap bubbles and show The experimental setup dedicated to the study of such large 2 that it becomes dominant above the critical size ` = a =e0, where bubbles is presented in Experimental Setup, before information p e0 is the mean thickness of the soap film and a = γb/ρg is the on Experimental Results and Model. The discussion on the asymp- capillary length (γb stands for vapor–liquid surface tension, and ρ totic shape and the analogy with inflated structures is presented stands for the liquid density). We first show experimentally that in Analogy with Inflatable Structures. large soap bubbles do not retain a spherical shape but flatten when increasing their size. A theoretical model is then developed Experimental Setup to account for this effect, predicting the shape based on mechan- The soap solution is prepared by mixing two volumes of Dreft© ical equilibrium. -

Soap Films: Statics and Dynamics
Soap Films: Statics and Dynamics Frederik Brasz January 8, 2010 1 Introduction Soap films and bubbles have long fascinated humans with their beauty. The study of soap films is believed to have started around the time of Leonardo da Vinci. Since then, research has proceeded in two distinct directions. On the one hand were the mathematicians, who were concerned with finding the shapes of these surfaces by minimizing their area, given some boundary. On the other hand, physical scientists were trying to understand the properties of soap films and bubbles, from the macroscopic behavior down to the molecular description. A crude way to categorize these two approaches is to call the mathematical shape- finding approach \statics" and the physical approach \dynamics", although there are certainly problems involving static soap films that require a physical description. This is my implication in this paper when I divide the discussion into statics and dynamics. In the statics section, the thickness of the film, concentration of surfactants, and gravity will all be quickly neglected, as the equilibrium shape of the surface becomes the only matter of importance. I will explain why for a soap film enclosing no volume, the mean curvature must everywhere be equal to zero, and why this is equivalent to the area of the film being minimized. For future reference, a minimal surface is defined as a two-dimensional surface with mean curvature equal to zero, so in general soap films take the shape of minimal surfaces. Carrying out the area minimization of a film using the Euler-Lagrange equation will result in a nonlinear partial differential equation known as Lagrange's Equation, which all minimal surfaces must satisfy. -
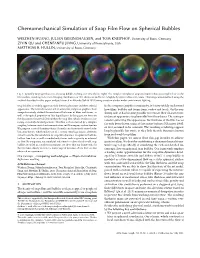
Chemomechanical Simulation of Soap Film Flow on Spherical Bubbles
Chemomechanical Simulation of Soap Film Flow on Spherical Bubbles WEIZHEN HUANG, JULIAN ISERINGHAUSEN, and TOM KNEIPHOF, University of Bonn, Germany ZIYIN QU and CHENFANFU JIANG, University of Pennsylvania, USA MATTHIAS B. HULLIN, University of Bonn, Germany Fig. 1. Spatially varying iridescence of a soap bubble evolving over time (left to right). The complex interplay of soap and water induces a complex flowonthe film surface, resulting in an ever changing distribution of film thickness and hence a highly dynamic iridescent texture. This image was simulated using the method described in this paper, and path-traced in Mitsuba [Jakob 2010] using a custom shader under environment lighting. Soap bubbles are widely appreciated for their fragile nature and their colorful In the computer graphics community, it is now widely understood appearance. The natural sciences and, in extension, computer graphics, have how films, bubbles and foams form, evolve and break. On the ren- comprehensively studied the mechanical behavior of films and foams, as dering side, it has become possible to recreate their characteristic well as the optical properties of thin liquid layers. In this paper, we focus on iridescent appearance in physically based renderers. The main pa- the dynamics of material flow within the soap film, which results in fasci- rameter governing this appearance, the thickness of the film, has so nating, extremely detailed patterns. This flow is characterized by a complex far only been driven using ad-hoc noise textures [Glassner 2000], coupling between surfactant concentration and Marangoni surface tension. We propose a novel chemomechanical simulation framework rooted in lu- or was assumed to be constant. -

Soap Bubbles for Volumetric Velocity Measurements in Air Flows
13th International Symposium on Particle Image Velocimetry – ISPIV 2019 Munich, Germany, July 22-24, 2019 Soap bubbles for volumetric velocity measurements in air flows Diogo Barros1;2∗, Yanchong Duan4, Daniel Troolin3 Ellen K. Longmire1, Wing Lai3 1 University of Minnesota, Aerospace Engineering and Mechanics, Minneapolis, MN 55455, USA 2 Aix-Marseille Universite,´ CNRS, IUSTI, Marseille, France 3 TSI, Incorporated, 500 Cardigan Rd., Shoreview, MN, USA 4 State Key Laboratory of Hydroscience and Engineering, Tsinghua University, Beijing 100084, China ∗ [email protected] Abstract The use of air-filled soap bubbles with diameter 10-30µm is demonstrated for volumetric velocimetry in air flows covering domains of 75-490cm3. The tracers are produced by a novel system that seeds high-density soap bubble streams for particle image velocimetry applications. Particle number density considerations, spatial resolution and response time scales are discussed in light of current seeding techniques for volumetric measurements. Finally, the micro soap bubbles are employed to measure the 3D velocity field in the wake of a sphere immersed in a turbulent boundary layer. 1 Introduction Volumetric velocimetry is a key enabler for understanding turbulent flows, which are inherently unsteady and three-dimensional. Multiple methods have been designed to measure 3D velocity fields accurately. In particular, particle image velocimetry (PIV) and particle tracking velocimetry (PTV) are now widely employed in various flow configurations (Discetti and Coletti, 2018). All of these techniques rely on imaging of discrete seeding tracers suspended in the flow. While larger seeding particles scatter more light from the illuminating source, smaller ones follow the flow more accurately and can resolve smaller scale variations. -

THE GEOMETRY of BUBBLES and FOAMS University of Illinois Department of Mathematics Urbana, IL, USA 61801-2975 Abstract. We Consi
THE GEOMETRY OF BUBBLES AND FOAMS JOHN M. SULLIVAN University of Illinois Department of Mathematics Urbana, IL, USA 61801-2975 Abstract. We consider mathematical models of bubbles, foams and froths, as collections of surfaces which minimize area under volume constraints. The resulting surfaces have constant mean curvature and an invariant notion of equilibrium forces. The possible singularities are described by Plateau's rules; this means that combinatorially a foam is dual to some triangulation of space. We examine certain restrictions on the combina- torics of triangulations and some useful ways to construct triangulations. Finally, we examine particular structures, like the family of tetrahedrally close-packed structures. These include the one used by Weaire and Phelan in their counterexample to the Kelvin conjecture, and they all seem useful for generating good equal-volume foams. 1. Introduction This survey records, and expands on, the material presented in the author's series of two lectures on \The Mathematics of Soap Films" at the NATO School on Foams (Cargese, May 1997). The first lecture discussed the dif- ferential geometry of constant mean curvature surfaces, while the second covered the combinatorics of foams. Soap films, bubble clusters, and foams and froths can be modeled math- ematically as collections of surfaces which minimize their surface area sub- ject to volume constraints. Each surface in such a solution has constant mean curvature, and is thus called a cmc surface. In Section 2 we examine a more general class of variational problems for surfaces, and then concen- trate on cmc surfaces. Section 3 describes the balancing of forces within a cmc surface.