Phylogenetic Relationships of Dasyuromorphian Marsupials Revisited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lindsay Masters
CHARACTERISATION OF EXPERIMENTALLY INDUCED AND SPONTANEOUSLY OCCURRING DISEASE WITHIN CAPTIVE BRED DASYURIDS Scott Andrew Lindsay A thesis submitted in fulfillment of requirements for the postgraduate degree of Masters of Veterinary Science Faculty of Veterinary Science University of Sydney March 2014 STATEMENT OF ORIGINALITY Apart from assistance acknowledged, this thesis represents the unaided work of the author. The text of this thesis contains no material previously published or written unless due reference to this material is made. This work has neither been presented nor is currently being presented for any other degree. Scott Lindsay 30 March 2014. i SUMMARY Neosporosis is a disease of worldwide distribution resulting from infection by the obligate intracellular apicomplexan protozoan parasite Neospora caninum, which is a major cause of infectious bovine abortion and a significant economic burden to the cattle industry. Definitive hosts are canid and an extensive range of identified susceptible intermediate hosts now includes native Australian species. Pilot experiments demonstrated the high disease susceptibility and the unexpected observation of rapid and prolific cyst formation in the fat-tailed dunnart (Sminthopsis crassicaudata) following inoculation with N. caninum. These findings contrast those in the immunocompetent rodent models and have enormous implications for the role of the dunnart as an animal model to study the molecular host-parasite interactions contributing to cyst formation. An immunohistochemical investigation of the dunnart host cellular response to inoculation with N. caninum was undertaken to determine if a detectable alteration contributes to cyst formation, compared with the eutherian models. Selective cell labelling was observed using novel antibodies developed against Tasmanian devil proteins (CD4, CD8, IgG and IgM) as well as appropriate labelling with additional antibodies targeting T cells (CD3), B cells (CD79b, PAX5), granulocytes, and the monocyte-macrophage family (MAC387). -

A Species-Level Phylogenetic Supertree of Marsupials
J. Zool., Lond. (2004) 264, 11–31 C 2004 The Zoological Society of London Printed in the United Kingdom DOI:10.1017/S0952836904005539 A species-level phylogenetic supertree of marsupials Marcel Cardillo1,2*, Olaf R. P. Bininda-Emonds3, Elizabeth Boakes1,2 and Andy Purvis1 1 Department of Biological Sciences, Imperial College London, Silwood Park, Ascot SL5 7PY, U.K. 2 Institute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, U.K. 3 Lehrstuhl fur¨ Tierzucht, Technical University of Munich, Alte Akademie 12, 85354 Freising-Weihenstephan, Germany (Accepted 26 January 2004) Abstract Comparative studies require information on phylogenetic relationships, but complete species-level phylogenetic trees of large clades are difficult to produce. One solution is to combine algorithmically many small trees into a single, larger supertree. Here we present a virtually complete, species-level phylogeny of the marsupials (Mammalia: Metatheria), built by combining 158 phylogenetic estimates published since 1980, using matrix representation with parsimony. The supertree is well resolved overall (73.7%), although resolution varies across the tree, indicating variation both in the amount of phylogenetic information available for different taxa, and the degree of conflict among phylogenetic estimates. In particular, the supertree shows poor resolution within the American marsupial taxa, reflecting a relative lack of systematic effort compared to the Australasian taxa. There are also important differences in supertrees based on source phylogenies published before 1995 and those published more recently. The supertree can be viewed as a meta-analysis of marsupial phylogenetic studies, and should be useful as a framework for phylogenetically explicit comparative studies of marsupial evolution and ecology. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Vpclistaug05 (.Pdf, 165.51KB)
Vertebrate Pests Committee List of Exotic Vertebrate Animals in Australia Rev Aug 2005 Contact Details Background Vertebrate Pests Committee Secretary All species on this list (excluding those with B or C c\o Land Protection annotations) form a definitive record of the non- Department of Natural Resources & Mines indigenous vertebrate mammals, birds, amphibians GPO Box 2454 and reptiles held in Australia under State and Territory BRISBANE QLD 4001 legislation. (It is the responsibility of the holder of an Tel. 07 3405 5540 individual animal to ensure that they are also Fax. 07 3405 5551 compliant with Commonwealth legislation relating to Mobile. the possession and quarantine of exotic animals.) Email. [email protected] This list should be used as a reference by the Vertebrate Pests Committee and Commonwealth, Sustainable Wildlife Industries State and Territory agencies in controlling the entry, Environment Australia movement and keeping of exotic vertebrate animals. GPO Box 787 CANBERRA ACT 2601 This list may be subject to change from time to time Tel. 02 6274 2880 (with VPC approval), to incorporate changes in the Fax. 02 6274 1921Email. [email protected] taxonomic name of species. It may also be changed where additions have been made through the legal importation of new exotic species into Australia under References the provisions of the Environmental Protection and Biodiversity Conservation Act 1999 and the Christidis, L. and Boles, W. E. 1994. The Taxonomy Quarantine Act 1908. and Species of Birds of Australia and its Territories. RAOU Monograph 2. Each species, unless otherwise stated, has so far only be subjected to a general assessment of the risk it Frost, N.C. -

Wildlife Matters Wildlife Conservancy
australian wildlife matters wildlife conservancy Spring 2009 Pungalina reveals one of Australia’s rarest mammals Carpentarian Pseudantechinus 2 australian saving australia’s threatened wildlife wildlife Pictograph conservancy Welcome to the Spring 2009 edition of Wildlife Matters. As this edition goes to print, we are in the process of fi nalising the acquisition of Bowra (see pages 4-5), a 14,000 the awc mission hectare property located in the heart of the Mulga Lands in Queensland. Bowra will The mission of Australian Wildlife Conservancy be our 21st sanctuary, bringing the AWC network to more than 2.56 million hectares (AWC) is the effective conservation of all (6.3 million acres). Australian animal species and the habitats in While the overall scale of the portfolio is impressive, it is not the number of properties or which they live. To achieve this mission, our hectares that really count. A more accurate measure of the value of the portfolio is the actions are focused on: number of species and ecosystems that occur within the AWC estate. In this respect, • Establishing a network of sanctuaries the statistics are even more impressive – for example, around 80% of all Australian which protect threatened wildlife and terrestrial bird species and over 60% of all terrestrial mammal species occur on one or ecosystems: AWC now manages 20 more of our sanctuaries. sanctuaries covering over 2.56 million The fact that our portfolio captures such a high percentage of Australia’s wildlife species hectares (6.3 million acres). refl ects a deliberate, science-based strategy to ensure that AWC invests in properties • Implementing practical, on-ground of the highest environmental value. -

Is Toxoplasma Gondii a Threat to the Conservation of Free-Ranging Australian Marsupial Populations?
International Journal for Parasitology: Parasites and Wildlife 5 (2016) 17e27 Contents lists available at ScienceDirect International Journal for Parasitology: Parasites and Wildlife journal homepage: www.elsevier.com/locate/ijppaw Review Is Toxoplasma gondii a threat to the conservation of free-ranging Australian marsupial populations? * Alison E. Hillman , Alan J. Lymbery, R.C. Andrew Thompson Murdoch University, 90 South St, Murdoch, WA 6150, Australia article info abstract Article history: It has often been asserted that Australian marsupial species are particularly susceptible to Toxoplasma Received 4 June 2015 gondii infection and to clinical toxoplasmosis following infection. This implicates T. gondii as a potential Received in revised form threat to marsupial population viability, and contrasts to what is known of T. gondii in populations of 7 December 2015 several other host species. We reviewed the literature, and found a lack of scientifically robust evidence Accepted 11 December 2015 addressing the occurrence of T. gondii infection in free-ranging populations of Australian marsupial species, and the impacts of the infection on population health. Key limitations included a lack of studies Keywords: in free-ranging marsupial populations, study findings susceptible to substantial chance influences, and Toxoplasma gondii fi fi Toxoplasmosis selection, misclassi cation and confounding biases. The lack of scienti cally robust data available on this Marsupials topic indicates that assertions that free-ranging populations of Australian marsupials are particularly Conservation susceptible to T. gondii infection and to toxoplasmosis are premature. The threat of T. gondii to the Epidemiology viability of free-ranging marsupial populations should therefore be regarded, at this stage, as a hypothesis. -
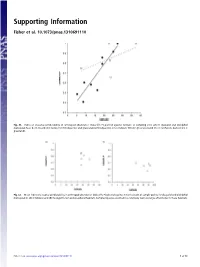
Supporting Information
Supporting Information Fisher et al. 10.1073/pnas.1310691110 Fig. S1. Index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against latitude of sampling sites where dasyurid and didelphid marsupials have been recorded in rainforest (filled points) and grassland (unfilled points). Lines indicate fitted regressions (solid line = rainforest, dashed line = grassland). Fig. S2. Mean index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against mean latitude of sample points for dasyurid and didelphid marsupials in (A) shrubland and (B) Eucalypt forest and woodland habitats. Sampled species occurred in a relatively narrow range of latitudes in these habitats. Fisher et al. www.pnas.org/cgi/content/short/1310691110 1of13 Fig. S3. Phylogeny of insectivorous marsupials with known life history data, based on ref. 1 with updates from ref. 2. 1. Cardillo M, Bininda-Emonds ORP, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264(1):11–31. 2. Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol Lett 12(6):538–549. Fisher et al. www.pnas.org/cgi/content/short/1310691110 2of13 Fisher et al. Table S1. Reproductive traits and diets of insectivorous marsupials Latitude (south) www.pnas.org/cgi/content/short/1310691110 Proportion Proportion Female for Habitat of of age species class for females males Male Breeding Copulation Litters Scrotal at first with species Genus species -

A New Species of False Antechinus (Marsupialia: Dasyuridae)
Rec. West. Aust. Mus. 1988,14(1): 35-59 A new species of false antechinus (Marsupialia~ Dasyuridae) from Western Australia, with remarks on the generic classification within the Parantechini D.]. Kitchener* and N. Caputit Abstract Pseudantechinus woolleyae sp. nov., frqm the Pilbara, Ashburton, Murchison and Little Sandy Desert regions of Western Australia, is described as a new species. It is pheneticaIly closest to Pseudantechinus macdonnellensis and phylogenetically closest to Pseudantechinus macdonnellensis, Pseudantechinus hilami and Pseudantechinus 'ningbing'. Introduction Tate (1947) in his taxonomic monograph on Dasyuridae placed Antechinus apicalis (Gray, 1842) in the monotypic genus Parantechinus. This was because it differed from all other Antechinus, except A. macdonnellensis (Spencer, 1896), in having an extreme reduction of the posterior premolars to single rooted vestiges. He placed A. macdonnellensis and A. mimulus (Thomas, 1906) in the genus Pseu dantechinus, because in addition to the reduction of their upper posterior pre molar, they totally lacked the corresponding lower tooth and also had an inflated alisphenoid and mastoid bulla. Ride (1964), in comments accompanying his description of Antechinus rosa mondae, considered that the morphology of his new species cast considerable doubt on the validity of the genera Parantechinus and Pseudantechinus. An techinus rosamondae had no posterior upper or lower premolar and an even more greatly inflated bulla than A. macdonnellensis. Rather than erect yet another mono typic genus, Ride argued for the return to the wider concept of Antechinus, noting that A. macdonnellensis spanned the range of dental characters of Antechinus (sensu latu) and that bulla inflation or its absence did not divide Antechinus into two groups. -

2014 Annual Reports of the Trustees, Standing Committees, Affiliates, and Ombudspersons
American Society of Mammalogists Annual Reports of the Trustees, Standing Committees, Affiliates, and Ombudspersons 94th Annual Meeting Renaissance Convention Center Hotel Oklahoma City, Oklahoma 6-10 June 2014 1 Table of Contents I. Secretary-Treasurers Report ....................................................................................................... 3 II. ASM Board of Trustees ............................................................................................................ 10 III. Standing Committees .............................................................................................................. 12 Animal Care and Use Committee .......................................................................... 12 Archives Committee ............................................................................................... 14 Checklist Committee .............................................................................................. 15 Conservation Committee ....................................................................................... 17 Conservation Awards Committee .......................................................................... 18 Coordination Committee ....................................................................................... 19 Development Committee ........................................................................................ 20 Education and Graduate Students Committee ....................................................... 22 Grants-in-Aid Committee -

Order Suborder Infraorder Superfamily Family
ORDER SUBORDER INFRAORDER SUPERFAMILY FAMILY SUBFAMILY TRIBE GENUS SUBGENUS SPECIES Monotremata Tachyglossidae Tachyglossus aculeatus Monotremata Tachyglossidae Zaglossus attenboroughi Monotremata Tachyglossidae Zaglossus bartoni Monotremata Tachyglossidae Zaglossus bruijni Monotremata Ornithorhynchidae Ornithorhynchus anatinus Didelphimorphia Didelphidae Caluromyinae Caluromys Caluromys philander Didelphimorphia Didelphidae Caluromyinae Caluromys Mallodelphys derbianus Didelphimorphia Didelphidae Caluromyinae Caluromys Mallodelphys lanatus Didelphimorphia Didelphidae Caluromyinae Caluromysiops irrupta Didelphimorphia Didelphidae Caluromyinae Glironia venusta Didelphimorphia Didelphidae Didelphinae Chironectes minimus Didelphimorphia Didelphidae Didelphinae Didelphis aurita Didelphimorphia Didelphidae Didelphinae Didelphis imperfecta Didelphimorphia Didelphidae Didelphinae Didelphis marsupialis Didelphimorphia Didelphidae Didelphinae Didelphis pernigra Didelphimorphia Didelphidae Didelphinae Didelphis virginiana Didelphimorphia Didelphidae Didelphinae Didelphis albiventris Didelphimorphia Didelphidae Didelphinae Gracilinanus formosus Didelphimorphia Didelphidae Didelphinae Gracilinanus emiliae Didelphimorphia Didelphidae Didelphinae Gracilinanus microtarsus Didelphimorphia Didelphidae Didelphinae Gracilinanus marica Didelphimorphia Didelphidae Didelphinae Gracilinanus dryas Didelphimorphia Didelphidae Didelphinae Gracilinanus aceramarcae Didelphimorphia Didelphidae Didelphinae Gracilinanus agricolai Didelphimorphia Didelphidae Didelphinae -
![June 2018 Newsletter [PDF 1 MB]Ecological Consulting News, A](https://docslib.b-cdn.net/cover/9422/june-2018-newsletter-pdf-1-mb-ecological-consulting-news-a-2589422.webp)
June 2018 Newsletter [PDF 1 MB]Ecological Consulting News, A
& Land for Wildlife and Garden for Wildlife Central Australia Newsletter June 2018 From the Land for Wildlife Coordinator In This Issue It’s hard to believe we are nearly half way through the year already! What a whirlwind few From the Land for Wildlife months. The Land for Wildlife team are over in WA for a couple of weeks so with the technology of mailchimp, this is being sent remotely while we enjoy a bushwalk through Boranup forest and get our feet wet in the cold waters of Hamelin Bay. The newsletter is a Coordinator • 1 quick one this month as a result, but there are plenty of great events coming up that we thought you should know about! The team will be back in time for the Alice Springs show, where we will have a stall to Ecological Consulting News • engage the masses on wildlife habitat conservation and restoration! Come and have a chat, buy a book and update us with your contact details if you’ve moved since we heard 2 from you last. We will be sharing the site with the Australian Plants Society this year that will be selling some native grasses, forbs, shrubs and trees—so pop on over to their table and buy yourself a plant to fill that gap in the garden. Don’t forget to check out the A Snippet From Significant Vegetation Maps on the Land for Wildlife website first to get an idea on what you could plant to suit your block. Trees » Lemon-scented Is there something native flora or fauna related that you want to learn about? Drop us an email and let us know and we’ll do the dirty groundwork and research to find out! Gum • 3 Junior Ranger • 4 Australian Plants Society Katherine Flora Brochure • 4 A Wasp making some moves to Pop Up Discovery Trail • 5 shift a Spider. -

This Article Appeared in a Journal Published by Elsevier. the Attached
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Physiology & Behavior 101 (2010) 389–393 Contents lists available at ScienceDirect Physiology & Behavior journal homepage: www.elsevier.com/locate/phb Basking behaviour in relation to energy use and food availability in one of the smallest marsupials Lisa Warnecke a,⁎, Elke Schleucher b, Fritz Geiser a a Centre for Behavioural and Physiological Ecology, Zoology, University of New England, Armidale, NSW 2351, Australia b Department of Ecology, Evolution and Diversity, Biosciences, Goethe-University, 60323 Frankfurt am Main, Germany article info abstract Article history: Although several mammals have been observed to bask in the sun, little is known about this behaviour or its Received 24 February 2010 energetic consequences. We investigated the importance of basking behaviour for one of the smallest Received in revised form 10 May 2010 marsupials, Planigale gilesi (9 g). Metabolic rates of captive planigales (n=6) exposed to simulated natural Accepted 9 July 2010 conditions with access to a radiant heat source were measured.