Compaction of Chromatin by Diverse Polycomb Group Proteins Requires Localized Regions of High Charge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dynamic Molecular Linkers of the Genome: the First Decade of SMC Proteins
Downloaded from genesdev.cshlp.org on October 8, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Dynamic molecular linkers of the genome: the first decade of SMC proteins Ana Losada1 and Tatsuya Hirano2,3 1Spanish National Cancer Center (CNIO), Madrid E-28029, Spain; 2Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA Structural maintenance of chromosomes (SMC) proteins in eukaryotes. The proposed actions of cohesin and con- are chromosomal ATPases, highly conserved from bac- densins offer a plausible, if not complete, explanation for teria to humans, that play fundamental roles in many the sudden appearance of thread-like “substances” (the aspects of higher-order chromosome organization and chromosomes) and their longitudinal splitting during dynamics. In eukaryotes, SMC1 and SMC3 act as the mitosis, first described by Walther Flemming (1882). core of the cohesin complexes that mediate sister chro- Remarkably, SMC proteins are conserved among the matid cohesion, whereas SMC2 and SMC4 function as three phyla of life, indicating that the basic strategy of the core of the condensin complexes that are essential chromosome organization may be evolutionarily con- for chromosome assembly and segregation. Another served from bacteria to humans. An emerging theme is complex containing SMC5 and SMC6 is implicated in that SMC proteins are dynamic molecular linkers of the DNA repair and checkpoint responses. The SMC com- genome that actively fold, tether, and manipulate DNA plexes form unique ring- or V-shaped structures with strands. Their diverse functions range far beyond chro- long coiled-coil arms, and function as ATP-modulated, mosome segregation, and involve nearly all aspects of dynamic molecular linkers of the genome. -

Specification in Embryonic Stem Cells Through Activation and Repression
Article Polycomb Regulates Mesoderm Cell Fate- Specification in Embryonic Stem Cells through Activation and Repression Mechanisms Graphical Abstract Authors Lluis Morey, Alexandra Santanach, Enrique Blanco, ..., Elphe` ge P. Nora, Benoit G. Bruneau, Luciano Di Croce Correspondence [email protected] (L.M.), [email protected] (L.D.C.) In Brief Morey et al. reveal that Mel18, a Polycomb-complex-associated protein, is an essential epigenetic regulator of cardiac differentiation. During directed differentiation of embryonic stem cells, Morey et al. find that Mel18-PRC1 complexes exchange subunits in a stage- specific manner and instruct sequential gene activation and repression programs to specify mesoderm fate, prevent alternate lineage commitment, and promote cardiac differentiation. Highlights Accession Numbers d Mel18 is required for PRC1 stability and maintenance of gene GSE67868 repression in ESCs d Mel18 is essential for ESC differentiation into early cardiac- mesoderm precursors d Different Mel18-PRC1 complexes are assembled during cardiac differentiation d Distinctive PRC1 complexes bind to highly active and repressed genes in MES cells Morey et al., 2015, Cell Stem Cell 17, 300–315 September 3, 2015 ª2015 Elsevier Inc. http://dx.doi.org/10.1016/j.stem.2015.08.009 Cell Stem Cell Article Polycomb Regulates Mesoderm Cell Fate-Specification in Embryonic Stem Cells through Activation and Repression Mechanisms Lluis Morey,1,2,6,* Alexandra Santanach,1,2 Enrique Blanco,1,2 Luigi Aloia,1,2 Elphe` ge P. Nora,3,4 Benoit G. Bruneau,3,4 -

2020 Program Book
PROGRAM BOOK Note that TAGC was cancelled and held online with a different schedule and program. This document serves as a record of the original program designed for the in-person meeting. April 22–26, 2020 Gaylord National Resort & Convention Center Metro Washington, DC TABLE OF CONTENTS About the GSA ........................................................................................................................................................ 3 Conference Organizers ...........................................................................................................................................4 General Information ...............................................................................................................................................7 Mobile App ....................................................................................................................................................7 Registration, Badges, and Pre-ordered T-shirts .............................................................................................7 Oral Presenters: Speaker Ready Room - Camellia 4.......................................................................................7 Poster Sessions and Exhibits - Prince George’s Exhibition Hall ......................................................................7 GSA Central - Booth 520 ................................................................................................................................8 Internet Access ..............................................................................................................................................8 -

Polycomb Group Proteins Ring1a/B Are Functionally Linked to the Core Transcriptional Regulatory Circuitry to Maintain ES Cell Identity Mitsuhiro Endoh1, Takaho A
Development ePress online publication date 13 March 2008 RESEARCH ARTICLE 1513 Development 135, 1513-1524 (2008) doi:10.1242/dev.014340 Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity Mitsuhiro Endoh1, Takaho A. Endo2, Tamie Endoh1, Yu-ichi Fujimura1, Osamu Ohara1, Tetsuro Toyoda2, Arie P. Otte3, Masaki Okano4, Neil Brockdorff5, Miguel Vidal1,6 and Haruhiko Koseki1,* The Polycomb group (PcG) proteins mediate heritable silencing of developmental regulators in metazoans, participating in one of two distinct multimeric protein complexes, the Polycomb repressive complexes 1 (PRC1) and 2 (PRC2). Although PRC2 has been shown to share target genes with the core transcription network, including Oct3/4, to maintain embryonic stem (ES) cells, it is still unclear whether PcG proteins and the core transcription network are functionally linked. Here, we identify an essential role for the core PRC1 components Ring1A/B in repressing developmental regulators in mouse ES cells and, thereby, in maintaining ES cell identity. A significant proportion of the PRC1 target genes are also repressed by Oct3/4. We demonstrate that engagement of PRC1 at target genes is Oct3/4-dependent, whereas engagement of Oct3/4 is PRC1-independent. Moreover, upon differentiation induced by Gata6 expression, most of the Ring1A/B target genes are derepressed and the binding of Ring1A/B to their target loci is also decreased. Collectively, these results indicate that Ring1A/B-mediated Polycomb -

Familial Cortical Myoclonus Caused by Mutation in NOL3 by Jonathan Foster Rnsseil DISSERTATION Submitted in Partial Satisfaction
Familial Cortical Myoclonus Caused by Mutation in NOL3 by Jonathan Foster Rnsseil DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Biomedical Sciences in the Copyright 2013 by Jonathan Foster Russell ii I dedicate this dissertation to Mom and Dad, for their adamantine love and support iii No man has earned the right to intellectual ambition until he has learned to lay his course by a star which he has never seen—to dig by the divining rod for springs which he may never reach. In saying this, I point to that which will make your study heroic. For I say to you in all sadness of conviction, that to think great thoughts you must be heroes as well as idealists. Only when you have worked alone – when you have felt around you a black gulf of solitude more isolating than that which surrounds the dying man, and in hope and in despair have trusted to your own unshaken will – then only will you have achieved. Thus only can you gain the secret isolated joy of the thinker, who knows that, a hundred years after he is dead and forgotten, men who never heard of him will be moving to the measure of his thought—the subtile rapture of a postponed power, which the world knows not because it has no external trappings, but which to his prophetic vision is more real than that which commands an army. -Oliver Wendell Holmes, Jr. iv ACKNOWLEDGMENTS I am humbled by the efforts of many, many others who were essential for this work. -

Bioinformatic Identification of Genes Suppressing Genome Instability
Bioinformatic identification of genes suppressing PNAS PLUS genome instability Christopher D. Putnama,b, Stephanie R. Allen-Solteroa,c, Sandra L. Martineza, Jason E. Chana,b, Tikvah K. Hayesa,1, and Richard D. Kolodnera,b,c,d,e,2 aLudwig Institute for Cancer Research, Departments of bMedicine and cCellular and Molecular Medicine, dMoores-University of California at San Diego Cancer Center, and eInstitute of Genomic Medicine, University of California School of Medicine at San Diego, La Jolla, CA 92093 Contributed by Richard D. Kolodner, September 28, 2012 (sent for review August 25, 2011) Unbiased forward genetic screens for mutations causing increased in concert to prevent genome rearrangements (reviewed in 12). gross chromosomal rearrangement (GCR) rates in Saccharomyces Modifications of the original GCR assay demonstrated that sup- cerevisiae are hampered by the difficulty in reliably using qualitative pression of GCRs mediated by segmental duplications and Ty GCR assays to detect mutants with small but significantly increased elements involves additional genes and pathways that do not GCR rates. We therefore developed a bioinformatic procedure using suppress single-copy sequence-mediated GCRs (13–15). Inter- genome-wide functional genomics screens to identify and prioritize estingly, homologs of some GCR-suppressing genes and pathways candidate GCR-suppressing genes on the basis of the shared drug suppress the development of cancer in mammals (16). Most of the sensitivity suppression and similar genetic interactions as known genes that suppress GCRs have been identified through a candi- GCR suppressors. The number of known suppressors was increased date gene approach. Some studies have screened collections of from 75 to 110 by testing 87 predicted genes, which identified un- arrayed S. -

PCGF2 Monoclonal Antibody (M07A), Clone 4E5
PCGF2 monoclonal antibody (M07A), clone 4E5 Catalog # : H00007703-M07A 規格 : [ 200 uL ] List All Specification Application Image Product Mouse monoclonal antibody raised against a partial recombinant Western Blot (Recombinant protein) Description: PCGF2. ELISA Immunogen: PCGF2 (AAH04858.1, 236 a.a. ~ 294 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Sequence: LTLATVPTPSEGTNTSGASECESVSDKAPSPATLPATSSSLPSPATPSH GSPSSHGPPA Host: Mouse Reactivity: Human Isotype: IgG2a Kappa Quality Control Antibody Reactive Against Recombinant Protein. Testing: Western Blot detection against Immunogen (32.23 KDa) . Storage Buffer: In ascites fluid Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Datasheet: Download Applications Western Blot (Recombinant protein) Protocol Download ELISA Gene Information Entrez GeneID: 7703 GeneBank BC004858 Page 1 of 2 2020/6/25 Accession#: Protein AAH04858.1 Accession#: Gene Name: PCGF2 Gene Alias: MEL-18,MGC10545,RNF110,ZNF144 Gene polycomb group ring finger 2 Description: Omim ID: 600346 Gene Ontology: Hyperlink Gene Summary: The protein encoded by this gene contains a RING finger motif and is similar to the polycomb group (PcG) gene products. PcG gene products form complexes via protein-protein interaction and maintain the transcription repression of genes involved in embryogenesis, cell cycles, and tumorigenesis. This protein was shown to act as a negative regulator of transcription and has tumor suppressor activity. The expression of this gene was detected in various tumor cells, but is limited in neural organs in normal tissues. Knockout studies in mice suggested that this protein may negatively regulate the expression of different cytokines, chemokines, and chemokine receptors, and thus plays an important role in lymphocyte differentiation and migration, as well as in immune responses. -

Identification of Novel Dna Damage Response Genes Using Functional Genomics
IDENTIFICATION OF NOVEL DNA DAMAGE RESPONSE GENES USING FUNCTIONAL GENOMICS by Michael Chang A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Biochemistry University of Toronto © Copyright by Michael Chang (2005) Identification of novel DNA damage response genes using functional genomics Doctor of Philosophy, 2005; Michael Chang; Department of Biochemistry, University of Toronto ABSTRACT The genetic information required for life is stored within molecules of DNA. This DNA is under constant attack as a result of normal cellular metabolic processes, as well as exposure to genotoxic agents. DNA damage left unrepaired can result in mutations that alter the genetic information encoded within DNA. Cells have consequently evolved complex pathways to combat damage to their DNA. Defects in the cellular response to DNA damage can result in genomic instability, a hallmark of cancer cells. Identifying all the components required for this response remains an important step in fully elucidating the molecular mechanisms involved. I used functional genomic approaches to identify genes required for the DNA damage response in Saccharomyces cerevisiae. I conducted a screen to identify genes required for resistance to a DNA damaging agent, methyl methanesulfonate, and identified several poorly characterized genes that are necessary for proper S phase progression in the presence of DNA damage. Among the genes identified, ESC4/RTT107 has since been shown to be essential for the resumption of DNA replication after DNA damage. Using genome-wide genetic interaction screens to identify genes that are required for viability in the absence of MUS81 and MMS4, two genes required for resistance to DNA damage, I helped identify ELG1, deletion of which causes DNA replication defects, genomic instability, and an inability to properly recover from DNA damage during S phase. -

Estrogen Exposure Overrides the Masculinizing Effect of Elevated
Díaz and Piferrer BMC Genomics (2017) 18:973 DOI 10.1186/s12864-017-4345-7 RESEARCH ARTICLE Open Access Estrogen exposure overrides the masculinizing effect of elevated temperature by a downregulation of the key genes implicated in sexual differentiation in a fish with mixed genetic and environmental sex determination Noelia Díaz1,2 and Francesc Piferrer1* Abstract Background: Understanding the consequences of thermal and chemical variations in aquatic habitats is of importance in a scenario of global change. In ecology, the sex ratio is a major population demographic parameter. So far, research that measured environmental perturbations on fish sex ratios has usually involved a few model species with a strong genetic basis of sex determination, and focused on the study of juvenile or adult gonads. However, the underlying mechanisms at the time of gender commitment are poorly understood. In an effort to elucidate the mechanisms driving sex differentiation, here we used the European sea bass, a fish species where genetics and environment (temperature) contribute equally to sex determination. Results: Here, we analyzed the transcriptome of developing gonads experiencing either testis or ovarian differentiation as a result of thermal and/or exogenous estrogen influences. These external insults elicited different responses. Thus, while elevated temperature masculinized genetic females, estrogen exposure was able to override thermal effects and resulted in an all-female population. A total of 383 genes were differentially expressed, with an overall downregulation in the expression of genes involved in both in testicular and ovarian differentiation when fish were exposed to Estradiol-17ß through a shutdown of the first steps of steroidogenesis. -

Control of Genome Integrity by RFC Complexes; Conductors of PCNA Loading Onto and Unloading from Chromatin During DNA Replication
Review Control of Genome Integrity by RFC Complexes; Conductors of PCNA Loading onto and Unloading from Chromatin during DNA Replication Yasushi Shiomi *and Hideo Nishitani * Graduate School of Life Science, University of Hyogo, Kamigori, Ako‐gun, Hyogo 678‐1297, Japan Correspondence: [email protected]‐hyogo.ac.jp (Y.S.); [email protected]‐hyogo.ac.jp (H.N.) Academic Editor: Eishi Noguchi Received: 28 November 2016; Accepted: 21 January 2017; Published: 26 January 2017 Abstract: During cell division, genome integrity is maintained by faithful DNA replication during S phase, followed by accurate segregation in mitosis. Many DNA metabolic events linked with DNA replication are also regulated throughout the cell cycle. In eukaryotes, the DNA sliding clamp, proliferating cell nuclear antigen (PCNA), acts on chromatin as a processivity factor for DNA polymerases. Since its discovery, many other PCNA binding partners have been identified that function during DNA replication, repair, recombination, chromatin remodeling, cohesion, and proteolysis in cell‐cycle progression. PCNA not only recruits the proteins involved in such events, but it also actively controls their function as chromatin assembles. Therefore, control of PCNA‐loading onto chromatin is fundamental for various replication‐coupled reactions. PCNA is loaded onto chromatin by PCNA‐loading replication factor C (RFC) complexes. Both RFC1‐RFC and Ctf18‐RFC fundamentally function as PCNA loaders. On the other hand, after DNA synthesis, PCNA must be removed from chromatin by Elg1‐RFC. Functional defects in RFC complexes lead to chromosomal abnormalities. In this review, we summarize the structural and functional relationships among RFC complexes, and describe how the regulation of PCNA loading/unloading by RFC complexes contributes to maintaining genome integrity. -

DNA Replication and Sister Chromatid Cohesion 1 (DSCC1)
Journal of Cancer 2019, Vol. 10 6142 Ivyspring International Publisher Journal of Cancer 2019; 10(24): 6142-6153. doi: 10.7150/jca.32339 Research Paper DNA Replication and Sister Chromatid Cohesion 1 (DSCC1) of the Replication Factor Complex CTF18- RFC is Critical for Colon Cancer Cell Growth Jong-Tae Kim1*, Hee Jun Cho1*, Sang Yoon Park1, Byung Moo Oh1,2, Yo Sep Hwang1,2, Kyoung Eun Baek1, Young-Ha Lee3, Hee Cheol Kim4 and Hee Gu Lee1,2 1. Immunotherapy Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Republic of Korea. 2. Department of Biomolecular Science, University of Science and Technology (UST), Daejeon, Republic of Korea. 3. Department of Infection Biology, Chungnam National University School of Medicine, Daejeon, Republic of Korea. 4. Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. *These authors contributed equally to this work. Corresponding authors: Hee Cheol Kim, M.D., Ph.D., Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. E-mail: [email protected] or Hee Gu Lee, Ph.D., Immunotherapy Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 34141, Republic of Korea. Tel: +82-42-860-4182; Fax: +82-42-860-4593; E-mail: [email protected] © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2018.12.17; Accepted: 2019.08.26; Published: 2019.10.15 Abstract DNA replication and sister chromatid cohesion 1 (DSCC1) combines with chromosome transmission-fidelity protein 18 (CTF18) to form a CTF18-DSCC1-CTF8 (CTF18-1-8) module, which in combination with CTF18-replication factor C (RFC) acts as a proliferating cell nuclear antigen (PCNA) loader during DNA replication-associated processes. -
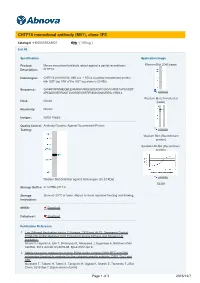
CHTF18 Monoclonal Antibody (M01), Clone 1F5
CHTF18 monoclonal antibody (M01), clone 1F5 Catalog # : H00063922-M01 規格 : [ 100 ug ] List All Specification Application Image Product Mouse monoclonal antibody raised against a partial recombinant Western Blot (Cell lysate) Description: CHTF18. Immunogen: CHTF18 (AAH18184, 886 a.a. ~ 975 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Sequence: GVHRPAPRNHEQRLEHIMRRAAREEQPEKDFFGRVVVRSTAVPSAGDT APEQDSVERRMGTAVGRSEVWFRFNEGVSNAVRRSLYIRDLL enlarge Western Blot (Transfected Host: Mouse lysate) Reactivity: Human Isotype: IgG2a Kappa Quality Control Antibody Reactive Against Recombinant Protein. Testing: enlarge Western Blot (Recombinant protein) Sandwich ELISA (Recombinant protein) enlarge Western Blot detection against Immunogen (35.53 KDa) . ELISA Storage Buffer: In 1x PBS, pH 7.4 Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Datasheet: Download Publication Reference 1. Two Different Replication Factor C Proteins, Ctf18 and RFC1, Separately Control PCNA-CRL4Cdt2-Mediated Cdt1 Proteolysis during S Phase and following UV Irradiation. Shiomi Y, Hayashi A, Ishii T, Shinmyozu K, Nakayama J, Sugasawa K, Nishitani H.Mol Cell Biol. 2012 Jun;32(12):2279-88. Epub 2012 Apr 9. 2. Stable interaction between the human PCNA loader complex Ctf18-RFC and DNA polymerase {epsilon} is mediated by the cohesion specific subunits, Ctf18, Dcc1 and Ctf8. Murakami T, Takano R, Takeo S, Taniguchi R, Ogawa K, Ohashi E, Tsurimoto T.J Biol Chem. 2010 Sep 7. [Epub ahead of print] Page 1 of 3 2016/12/7 Applications Western Blot (Cell lysate) CHTF18 monoclonal antibody (M01), clone 1F5 Western Blot analysis of CHTF18 expression in HeLa ( Cat # L013V1 ). Protocol Download Western Blot (Transfected lysate) Western Blot analysis of CHTF18 expression in transfected 293T cell line by CHTF18 monoclonal antibody (M01), clone 1F5.