Tablets 20, 40, and 80 Mg WARNING LASIX (Furosemide)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

268 Part 522—Implantation Or Injectable Dosage Form
§ 520.2645 21 CFR Ch. I (4–1–18 Edition) (ii) Indications for use. For the control 522.82 Aminopropazine. of American foulbrood (Paenibacillus 522.84 Beta-aminopropionitrile. larvae). 522.88 Amoxicillin. 522.90 Ampicillin injectable dosage forms. (iii) Limitations. The drug should be 522.90a Ampicillin trihydrate suspension. fed early in the spring or fall and con- 522.90b Ampicillin trihydrate powder for in- sumed by the bees before the main jection. honey flow begins, to avoid contamina- 522.90c Ampicillin sodium. tion of production honey. Complete 522.144 Arsenamide. treatments at least 4 weeks before 522.147 Atipamezole. main honey flow. 522.150 Azaperone. 522.161 Betamethasone. [40 FR 13838, Mar. 27, 1975, as amended at 50 522.163 Betamethasone dipropionate and FR 49841, Dec. 5, 1985; 59 FR 14365, Mar. 28, betamethasone sodium phosphate aque- 1994; 62 FR 39443, July 23, 1997; 68 FR 24879, ous suspension. May 9, 2003; 70 FR 69439, Nov. 16, 2005; 73 FR 522.167 Betamethasone sodium phosphate 76946, Dec. 18, 2008; 75 FR 76259, Dec. 8, 2010; and betamethasone acetate. 76 FR 59024, Sept. 23, 2011; 77 FR 29217, May 522.204 Boldenone. 17, 2012; 79 FR 37620, July 2, 2014; 79 FR 53136, 522.224 Bupivacaine. Sept. 8, 2014; 79 FR 64116, Oct. 28, 2014; 80 FR 522.230 Buprenorphine. 34278, June 16, 2015; 81 FR 48702, July 26, 2016] 522.234 Butamisole. 522.246 Butorphanol. § 520.2645 Tylvalosin. 522.275 N-Butylscopolammonium. 522.300 Carfentanil. (a) Specifications. Granules containing 522.304 Carprofen. 62.5 percent tylvalosin (w/w) as 522.311 Cefovecin. -

Sample Chapter
LCMS_Chap09 (JB-D) 8/5/06 3:14 pm Page 193 9 LC-MS in doping control Detlef Thieme Introduction adjacent fields with similar analytical prospects, like veterinary residue control (predominantly dealing with identification of growth promoters Definition of doping in various matrices), forensic sciences (the major- ity of doping-relevant substances are scheduled Doping analysis comprises a diversity of sub- as controlled substances in most countries), stance classes with different pharmaceutical and environmental analysis (e.g. steroids in waste chemical properties. Therefore, the discussion of water) or clinical chemistry (e.g. due to the the suitability of liquid chromatography-mass increasing relevance of steroid hormone replace- spectrometry (LC-MS) in doping analysis needs ment therapy). to distinguish various categories. This chapter describes the key fields of appli- According to its formal definition, a doping cation of LC-MS in routine doping control (i.e. violation in sports can be caused by various screening analysis, confirmation and quantifica- events, e.g.: tion of positive results) extra to particular • the detection of a prohibited substance or research activities. The latter are focused on the metabolites or markers of that substance (as intended technical improvements (e.g. extension defined by the recent document [1] of the of detection time windows, reduction of turn- World Anti-Doping Agency [WADA]) in the around times and costs) of conventional analyti- athlete’s specimen cal procedures and, in particular, on the detection • the use of prohibited substances or methods of prohibited substances that cannot be ade- • possession or trafficking prohibited substances quately identified so far (e.g. -

DIURETICS Diuretics Are Drugs That Promote the Output of Urine Excreted by the Kidneys
DIURETICS Diuretics are drugs that promote the output of urine excreted by the Kidneys. The primary action of most diuretics is the direct inhibition of Na+ transport at one or more of the four major anatomical sites along the nephron, where Na+ reabsorption takes place. The increased excretion of water and electrolytes by the kidneys is dependent on three different processes viz., glomerular filtration, tubular reabsorption (active and passive) and tubular secretion. Diuretics are very effective in the treatment of Cardiac oedema, specifically the one related with congestive heart failure. They are employed extensively in various types of disorders, for example, nephritic syndrome, diabetes insipidus, nutritional oedema, cirrhosis of the liver, hypertension, oedema of pregnancy and also to lower intraocular and cerebrospinal fluid pressure. Therapeutic Uses of Diuretics i) Congestive Heart Failure: The choice of the diuretic would depend on the severity of the disorder. In an emergency like acute pulmonary oedema, intravenous Furosemide or Sodium ethacrynate may be given. In less severe cases. Hydrochlorothiazide or Chlorthalidone may be used. Potassium-sparing diuretics like Spironolactone or Triamterene may be added to thiazide therapy. ii) Essential hypertension: The thiazides usually sever as primary antihypertensive agents. They may be used as sole agents in patients with mild hypertension or combined with other antihypertensives in more severe cases. iii) Hepatic cirrhosis: Potassium-sparing diuretics like Spironolactone may be employed. If Spironolactone alone fails, then a thiazide diuretic can be added cautiously. Furosemide or Ethacrymnic acid may have to be used if the oedema is regractory, together with spironolactone to lessen potassium loss. Serum potassium levels should be monitored periodically. -

Uniform Classification Guidelines for Foreign Substances and Recommended Penalties Model Rule
DRUG TESTING STANDARDS AND PRACTICES PROGRAM. Uniform Classification Guidelines for Foreign Substances And Recommended Penalties Model Rule. January, 2018 (V.13.4) Ó ASSOCIATION OF RACING COMMISSIONERS INTERNATIONAL – 2018. Association of Racing Commissioners International 1510 Newtown Pike, Lexington, Kentucky, United States www.arci.com Page 1 of 61 Preamble to the Uniform Classification Guidelines of Foreign Substances The Preamble to the Uniform Classification Guidelines was approved by the RCI Drug Testing and Quality Assurance Program Committee (now the Drug Testing Standards and Practices Program Committee) on August 26, 1991. Minor revisions to the Preamble were made by the Drug Classification subcommittee (now the Veterinary Pharmacologists Subcommittee) on September 3, 1991. "The Uniform Classification Guidelines printed on the following pages are intended to assist stewards, hearing officers and racing commissioners in evaluating the seriousness of alleged violations of medication and prohibited substance rules in racing jurisdictions. Practicing equine veterinarians, state veterinarians, and equine pharmacologists are available and should be consulted to explain the pharmacological effects of the drugs listed in each class prior to any decisions with respect to penalities to be imposed. The ranking of drugs is based on their pharmacology, their ability to influence the outcome of a race, whether or not they have legitimate therapeutic uses in the racing horse, or other evidence that they may be used improperly. These classes of drugs are intended only as guidelines and should be employed only to assist persons adjudicating facts and opinions in understanding the seriousness of the alleged offenses. The facts of each case are always different and there may be mitigating circumstances which should always be considered. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Mechanisms of Sevoflurane-Induced Myocardial Preconditioning In
Anesthesiology 2003; 99:27–33 © 2003 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Mechanisms of Sevoflurane-induced Myocardial Preconditioning in Isolated Human Right Atria In Vitro Alexandra Yvon, B.Sc.,* Jean-Luc Hanouz, M.D, Ph.D.,† Benoît Haelewyn, B.Sc.,* Xavier Terrien, B.Sc.,* Massimo Massetti, M.D.,‡ Gérard Babatasi, M.D., Ph.D.,‡ André Khayat, M.D.,§ Pierre Ducouret, Ph.D., Henri Bricard, M.D.,# Jean-Louis Gérard, M.D., Ph.D.** Background: The authors examined the role of adenosine postischemic contractile function,5–7 and metabolic triphosphate–sensitive potassium channels and adenosine A 7 1 function. Although the mechanisms involved in anes- receptors in sevoflurane-induced preconditioning on isolated thetic preconditioning remain incompletely understood, human myocardium. Methods: The authors recorded isometric contraction of hu- protein kinase C, adenosine triphosphate–sensitive po- man right atrial trabeculae suspended in oxygenated Tyrode’s tassium (KATP) channels, and adenosine A1 receptors Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/99/1/27/408853/0000542-200307000-00008.pdf by guest on 02 October 2021 solution (34°C; stimulation frequency, 1 Hz). In all groups, a seem to play a key role.1,2,5,6 However, recent studies 30-min hypoxic period was followed by 60 min of reoxygenation. suggest that volatile anesthetic–induced myocardial pre- Seven minutes before hypoxia reoxygenation, muscles were ex- posed to 4 min of hypoxia and 7 min of reoxygenation or 15 min conditioning may be dependent on species and experi- 5,8 of sevoflurane at concentrations of 1, 2, and 3%. In separate mental models. -

FAST FACTS and CONCEPTS #20 OPIOID DOSE ESCALATION in END-OF-LIFE CARE David E Weissman MD, Andrew Kamell MD Background a Comm
FAST FACTS AND CONCEPTS #20 OPIOID DOSE ESCALATION IN END-OF-LIFE CARE David E Weissman MD, Andrew Kamell MD Background A common question from trainees is “How fast, and by how much, can opioids be safely dose escalated in terminally ill patients?” Consider the analogy of furosemide (Lasix) when discussing this topic. An increase in Lasix from 10 mg to 11 mg is unheard of, yet that is precisely what often happens with opioids, especially parenteral infusions. For example, increasing a morphine infusion from 1 to 2 mg/hr is a 100% does increase; while going from 5 to 6 mg/hr is only a 20% increase. Yet, many orders are written, “increase drip by 1 mg/hr, titrate to comfort.” Some hospitals and nursing units even have this as a standing order or nursing policy. Such titration orders are unlikely to lead to be effective. Key clinical considerations for appropriate opioid escalation: 1. Is an opioid dose escalation appropriate? To answer that question, clinicians need to consider: a. Is the patient tolerating opioids well with little adverse effects? b. Is the pain or dyspnea expected to continue at a similar or progressive pattern (particularly relevant for scheduled medications or infusions) or might it be temporary? c. Is the source of discomfort opioid responsive? d. Is there evidence of hyperalgesia or neurotoxicity from opioids? See Fast Facts #57,58, and 142. e. Is the pain related to a chronic, non-malignant source in which the patient has an extended prognosis? If so, then aggressive opioid titrations may not be the best long-term solution. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

2021 Formulary List of Covered Prescription Drugs
2021 Formulary List of covered prescription drugs This drug list applies to all Individual HMO products and the following Small Group HMO products: Sharp Platinum 90 Performance HMO, Sharp Platinum 90 Performance HMO AI-AN, Sharp Platinum 90 Premier HMO, Sharp Platinum 90 Premier HMO AI-AN, Sharp Gold 80 Performance HMO, Sharp Gold 80 Performance HMO AI-AN, Sharp Gold 80 Premier HMO, Sharp Gold 80 Premier HMO AI-AN, Sharp Silver 70 Performance HMO, Sharp Silver 70 Performance HMO AI-AN, Sharp Silver 70 Premier HMO, Sharp Silver 70 Premier HMO AI-AN, Sharp Silver 73 Performance HMO, Sharp Silver 73 Premier HMO, Sharp Silver 87 Performance HMO, Sharp Silver 87 Premier HMO, Sharp Silver 94 Performance HMO, Sharp Silver 94 Premier HMO, Sharp Bronze 60 Performance HMO, Sharp Bronze 60 Performance HMO AI-AN, Sharp Bronze 60 Premier HDHP HMO, Sharp Bronze 60 Premier HDHP HMO AI-AN, Sharp Minimum Coverage Performance HMO, Sharp $0 Cost Share Performance HMO AI-AN, Sharp $0 Cost Share Premier HMO AI-AN, Sharp Silver 70 Off Exchange Performance HMO, Sharp Silver 70 Off Exchange Premier HMO, Sharp Performance Platinum 90 HMO 0/15 + Child Dental, Sharp Premier Platinum 90 HMO 0/20 + Child Dental, Sharp Performance Gold 80 HMO 350 /25 + Child Dental, Sharp Premier Gold 80 HMO 250/35 + Child Dental, Sharp Performance Silver 70 HMO 2250/50 + Child Dental, Sharp Premier Silver 70 HMO 2250/55 + Child Dental, Sharp Premier Silver 70 HDHP HMO 2500/20% + Child Dental, Sharp Performance Bronze 60 HMO 6300/65 + Child Dental, Sharp Premier Bronze 60 HDHP HMO -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -
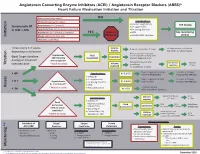
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

218 Part 522—Implantation Or Injectable Dosage Form
Pt. 522 21 CFR Ch. I (4–1–07 Edition) by Mycoplasma gallisepticum sensitive 522.82 Aminopropazine fumarate sterile so- to tylosin. lution injection. (iii) Limitations. Do not use in layers 522.84 Beta-aminopropionitrile fumarate. 522.88 Sterile amoxicillin trihydrate for sus- producing eggs for human consump- pension. tion; administer from 2 to 5 days as 522.90 Ampicillin implantation and sole source of drinking water; treated injectible dosage forms. turkeys should consume enough medi- 522.90a Ampicillin trihydrate sterile suspen- cated drinking water to provide 60 mil- sion. ligrams of tylosin per pound of body 522.90b Ampicillin trihydrate for sterile sus- weight per day; prepare a fresh solu- pension. tion every 3 days; when sinus swelling 522.90c Ampicillin sodium for aqueous injec- tion. is present, inject the sinus with tylosin 522.144 Arsenamide sodium aqueous injec- injectable simultaneously with the tion. drinking water treatment; do not ad- 522.147 Atipamezole. minister within 5 days of slaughter. 522.150 Azaperone injection. (3) Swine—(i) Amount. 0.25 gram per 522.161 Betamethasone acetate and gallon. betamethasone disodium phosphate aque- (ii) Indications for use. For the control ous suspension. 522.163 Betamethasone dipropionate and and treatment of swine dysentery betamethasone sodium phosphate aque- (bloody scours) caused by pathogens ous suspension. sensitive to tylosin. 522.204 Boldenone. (iii) Limitations. As only source of 522.234 Butamisole hydrochloride. drinking water for 3 to 10 days, depend- 522.246 Butorphanol tartrate injection. ing on the severity of the condition 522.275 N-Butylscopolammonium bromide. being treated: mix fresh solution daily; 522.311 Carfentanil citrate injection.