Thermodynamics for Dummies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cryogenic Refrigeration Using an Acoustic Stirling Expander
CRYOGENIC REFRIGERATION USING AN ACOUSTIC STIRLING EXPANDER Masters Thesis by Nick Emery Department of Mechanical Engineering, University of Canterbury Christchurch, New Zealand Abstract A single-stage pulse tube cryocooler was designed and fabricated to provide cooling at 50 K for a high temperature superconducting (HTS) magnet, with a nominal electrical input frequency of 50 Hz and a maximum mean helium working gas pressure of 2.5 MPa. Sage software was used for the thermodynamic design of the pulse tube, with an initially predicted 30 W of cooling power at 50 K, and an input indicated power of 1800 W. Sage was found to be a useful tool for the design, and although not perfect, some correlation was established. The fabricated pulse tube was closely coupled to a metallic diaphragm pressure wave generator (PWG) with a 60 ml swept volume. The pulse tube achieved a lowest no-load temperature of 55 K and provided 46 W of cooling power at 77 K with a p-V input power of 675 W, which corresponded to 19.5% of Carnot COP. Recommendations included achieving the specified displacement from the PWG under the higher gas pressures, design and development of a more practical co-axial pulse tube and a multi-stage configuration to achieve the power at lower temperatures required by HTS. ii Acknowledgements The author acknowledges: His employer, Industrial Research Ltd (IRL), New Zealand, for the continued support of this work, Alan Caughley for all his encouragement, help and guidance, New Zealand’s Foundation for Research, Science and Technology for funding, University of Canterbury – in particular Alan Tucker and Michael Gschwendtner for their excellent supervision and helpful input, David Gedeon for his Sage software and great support, Mace Engineering for their manufacturing assistance, my wife Robyn for her support, and HTS-110 for creating an opportunity and pathway for the commercialisation of the device. -

Chapter 9, Problem 16. an Air-Standard Cycle Is Executed in A
COSMOS: Complete Online Solutions Manual Organization System Chapter 9, Problem 16. An air-standard cycle is executed in a closed system and is composed of the following four processes: 1-2 Isentropic compression from 100 kPa and 27°C to 1 MPa 2-3 P = constant heat addition in amount of 2800 kJ/kg 3-4 v = constant heat rejection to 100 kPa 4-1 P = constant heat rejection to initial state (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the maximum temperature in the cycle. (c) Determine the thermal efficiency. Assume constant specific heats at room temperature. * Problems designated by a “C” are concept questions, and students are encouraged to answer them all. Problems designated by an “E” are in English units, and the SI users can ignore them. Problems with the are solved using EES, and complete solutions together with parametric studies are included on the enclosed DVD. Problems with the are comprehensive in nature and are intended to be solved with a computer, preferably using the EES software that accompanies this text. Thermodynamics: An Engineering Approach, 5/e, Yunus Çengel and Michael Boles, © 2006 The McGraw-Hill Companies. COSMOS: Complete Online Solutions Manual Organization System Chapter 9, Problem 37. The compression ratio of an air-standard Otto cycle is 9.5. Prior to the isentropic compression process, the air is at 100 kPa, 35°C, and 600 cm3. The temperature at the end of the isentropic expansion process is 800 K. Using specific heat values at room temperature, determine (a) the highest temperature and pressure in the cycle; (b) the amount of heat transferred in, in kJ; (c) the thermal efficiency; and (d) the mean effective pressure. -

In-Cylinder Heat Transfer in an Ericsson Engine Prototype
International Conference on Renewable Energies and Power Quality (ICREPQ’13) Bilbao (Spain), 20th to 22th March, 2013 exÇxãtuÄx XÇxÜzç tÇw cÉãxÜ dâtÄ|àç ]ÉâÜÇtÄ (RE&PQJ) ISSN 2172-038 X, No.11, March 2013 In-Cylinder Heat Transfer in an Ericsson Engine Prototype A. FULA 1,2, P. STOUFFS 1 and F.SIERRA2 1 Laboratoire de Thermique, Energétique et Procédés Université de Pau et des Pays de l´Adour 64000, Pau, France. e-mail: [email protected] 2 Facultad de Ingenieria – Laboratorio Máquinas Térmicas y Energias Renovables Universidad Nacional de Colombia - Carrera 30 No 45 a 03 Edificio 401, Bogotá, Colombia. Phone 3165000 xt 11130, e-mail: [email protected] [email protected] Abstract. An Ericsson engine is an external heat supply Qh Qc engine working according to a Joule thermodynamic cycle. It is based on reciprocating piston-cylinder machines. Such engines are especially interesting for low power solar energy conversion and micro-CHP from conventional fossil fuels or from biomass. H RK An Ericsson engine prototype has been designed and realized. Due to the geometrical configuration of the compression space of this prototype, in-cylinder fluid wall heat transfer can be important. The influence of this heat transfer on the engine performance is modelled and the main results are presented. E C The experimental setup designed to study the in-cylinder heat Fig. 1. Principle of the Stirling engine. transfer is presented. Q Key words K c Solar energy conversion, biomass energy conversion, H R Ericsson engine, hot air engines, distributed generation, cylinder wall heat transfer. -

A Novel Thermomechanical Energy Conversion Cycle ⇑ Ian M
Applied Energy 126 (2014) 78–89 Contents lists available at ScienceDirect Applied Energy journal homepage: www.elsevier.com/locate/apenergy A novel thermomechanical energy conversion cycle ⇑ Ian M. McKinley, Felix Y. Lee, Laurent Pilon Mechanical and Aerospace Engineering Department, Henry Samueli School of Engineering and Applied Science, University of California, Los Angeles, Los Angeles, CA 90095, USA highlights Demonstration of a novel cycle converting thermal and mechanical energy directly into electrical energy. The new cycle is adaptable to changing thermal and mechanical conditions. The new cycle can generate electrical power at temperatures below those of other pyroelectric power cycles. The new cycle can generate larger electrical power than traditional mechanical cycles using piezoelectric materials. article info abstract Article history: This paper presents a new power cycle for direct conversion of thermomechanical energy into electrical Received 21 May 2013 energy performed on pyroelectric materials. It consists sequentially of (i) an isothermal electric poling Received in revised form 18 February 2014 process performed under zero stress followed by (ii) a combined uniaxial compressive stress and heating Accepted 26 March 2014 process, (iii) an isothermal electric de-poling process under uniaxial stress, and finally (iv) the removal of compressive stress during a cooling process. The new cycle was demonstrated experimentally on [001]-poled PMN-28PT single crystals. The maximum power and energy densities obtained were Keywords: 41 W/L and 41 J/L/cycle respectively for cold and hot source temperatures of 22 and 130 °C, electric field Pyroelectric materials between 0.2 and 0.95 MV/m, and with uniaxial load of 35.56 MPa at frequency of 1 Hz. -

Lecture 3 Thermodynamic Principles of Energy Conversion
Thermodynamic principles of energy conversion Md. Mizanur Rahman School of Mechanical Engineering Universiti Teknologi Malaysia 1 Introduction • Mechanical and electrical power developed from the combustion of fossil fuels or the fission of nuclear fuel or renewable sources • This released energy is never lost but is transformed into other forms • This conservation of energy is explicitly expressed by the first law of thermodynamics • The work of an engine cannot be the same as the energy available in the fuel sources • Second law of thermodynamics is to meet to happen the process 2 Forms of energy • Energy can exist in numerous forms such as thermal, mechanical, kinetic, potential, electric, magnetic, chemical, and nuclear, and their sum constitutes the total energy, E of a system. • Mechanical Energy • Kinetic energy, KE: The energy that a system possesses as a result of its motion relative to some reference frame, KE=1/2mV2. • Potential energy, PE: The energy that a system possesses as a result of its elevation in a gravitational field, PE=mgh • For a moving body, E=PE+KE remains constant 3 Internal energy, U: The sum of all the microscopic forms of energy. • For gases, molecules are so widely separated in space that they may be considered to be moving independently of each other, each possessing a distinct total energy. • In the case of liquids or solids, each molecule is under the influence of forces exerted by nearby molecules • Changes in internal energy are measurable by changes in temperature, pressure, and density. 4 • Chemical energy: The internal energy associated with the atomic bonds in a molecule. -

Intro-Propulsion-Lect-18
18 1 Lect-18 In this lecture ... • Stirling and Ericsson cycles • Brayton cycle: The ideal cycle for gas- turbine engines • The Brayton cycle with regeneration • The Brayton cycle with intercooling, reheating and regeneration • Rankine cycle: The ideal cycle for vapour power cycles 2 Prof. Bhaskar Roy, Prof. A M Pradeep, Department of Aerospace, IIT Bombay Lect-18 Stirling and Ericsson cycles • The ideal Otto and Diesel cycles are internally reversible, but not totally reversible. • Hence their efficiencies will always be less than that of Carnot efficiency. • For a cycle to approach a Carnot cycle, heat addition and heat rejection must take place isothermally. • Stirling and Ericsson cycles comprise of isothermal heat addition and heat rejection. 3 Prof. Bhaskar Roy, Prof. A M Pradeep, Department of Aerospace, IIT Bombay Lect-18 Regeneration Working fluid • Both these cycles also have a regeneration process. • Regeneration, a process during which heat is Energy transferred to a thermal energy storage device Energy (called a regenerator) during one part of the cycle and is transferred back to the working fluid during Concept of a regenerator another part of the cycle. 4 Prof. Bhaskar Roy, Prof. A M Pradeep, Department of Aerospace, IIT Bombay Lect-18 Stirling cycle • Consists of four totally reversible processes: – 1-2 T = constant, expansion (heat addition from the external source) – 2-3 v = constant, regeneration (internal heat transfer from the working fluid to the regenerator) – 3-4 T= constant, compression (heat rejection to the external sink) – 4-1 v = constant, regeneration (internal heat transfer from the regenerator back to the working fluid) 5 Prof. -

Turbo Brayton for Space
Turbo Brayton for Space Julien TANCHON Page 1 Objectives of the talk : - A quick reminder of the thermodynamic cycles - Review of the Turbo-Brayton cycles and general concept - Interest of this technology for space applications - Technologies implemented within Turbo-Brayton for space - Examples of Turbo-Brayton activities Page 2 Thermodynamic cycles The main types of cryogenic coolers (or cryocooler) commonly used to achieve cryogenic temperatures are classified in 2 categories Heat exchangers Regenerators Page 3 Thermodynamic cycles The main types of cryogenic coolers (or cryocooler) commonly used to achieve cryogenic temperatures are: Joule-Thomson Brayton Stirling Pulse Tube Gifford-McMahon • Operating temperature range : 1.6K to 300K • Cooling power range: from few mW up to hundreds of W • Reduced thermodynamic efficiency due to isenthalpic expansion • Multi-stage cooling possible • High pressure compressor complexe Page 4 Thermodynamic cycles The main types of cryogenic coolers (or cryocooler) commonly used to achieve cryogenic temperatures are: Joule-Thomson Brayton Stirling Pulse Tube Gifford-McMahon • Operating temperature range : 4K to 300K • Cooling power range: from few mW up to kW • High efficiency, particularly for high capacity cooler • Very compact due to the motor compacity • Important lifetime and reliability • Distributed load and intermediate cooling stages • Limit for manufacturing small compressor and turbine wheels Page 5 Thermodynamic cycles The main types of cryogenic coolers (or cryocooler) commonly used to achieve -

Bibliography
Bibliography Cook, E.,Man, Energy, Society (Freeman, San Francisco, 1976) Fast, J. D., Entropy, 2nd ed. (Macmillan, l.ondon, 1970) Goodger, E. M. Principles of Engineering Thermodynamics (Macmillan, l.ondon, 1974) Hatsopoulos, G. N., and Keenan, J. H., Principles ofGeneral Thermodynamics (Wiley, New York, 1965) Jones, J. B., and Hawkins, G. A., Engineering Thermodynamics (Wiley, New York, 1960) Keenan, J. H., Thermodynamics (Wiley, New York, 1941) Lee, J. F., and Sears, F. W., Thermodynamics (Addison-Wesley, London, 1962) Obert, E. F., Thermodynamics (McGraw-Hill, New York, 1948) Soo, S. L., Thermodynamics ofEngineering Science (Prentice-Hall, Englewood Oiffs, N.J., 1958) Spalding, D. B., and Cole, E. H., Engineering Thermodynamics, 3rd ed. (Edward Arnold,l.ondon, 1973) Turton, J. S., Macroscopic Thermodynamics (Wiley, l.ondon, 1973) Tyldesley, J. R., An Introduction to Applied Thermodynamics and Energy Conversion (l.ongman, New York, 1977) Van Wylen, G. J., and Sonntag, R., Fundamentals ofClassical Thermodyamics (Wiley, New York, 1967) Wallace, F. J. and Linning, W. A., Basic Engineering Thermodynamics (Pitman, Bath,1968) Wark, K., Thermodynamics (McGraw-Hill, New York, 1966) 152 Index Absolute zero temperature 58 Darreus function 86 Actua1 gases 19 Dead state 90, 96 Adiabatic friction1ess process 33 Definitions 1 Availability 85,133,147 Degradation of energy 50 closed system 92,93,94, Derived properties 85,86 95,100,121 Diesel cycle 73 Availabilityaccounting 110,114,117 Disordered energy 3, 10 Availability concept 89 Availability function -

A Study of Design Principles for Refrigerators for Low-Power Cryoelectronic Devices
NATL INST. OF STAND & TEC[ NSST PUBLICATIONS A 1 1 1 5 TaZflfl3 <*** 0F c% IN NBS TECHNICAL NOTE 1049 \ ^^ J? **»EAU O* ^ U.S. DEPARTMENT OF COMMERCBNational Bureau of Standards NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system ol physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes -

Applications of Closed-Cycle Cryocoolers to Small Superconducting Devices April 1978 Proceedings of a Conference Held at the National Biireau 6
A111D3 DLbSbfi '^*™illiinLi™.?I'!},!:'.9i!'SP.S&.^ R.I.C. «KW3t??..„ SPECIAL PUBLICATION 508 U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards 1 osea- 0 Small l7Q tioMl Bureau of Staruiards MAY 1 i 1978 ^ Applications of Closed-Cycle Cryocoolers to ^0 Small Superconducting Devices Proceedings of a Conference Held at the National Bureau of Standards, Boulder, Colorado October 3-4, 1977 Edited by James E. Zimmerman and Thomas M. Flynn Cryogenics Division Institute for Basic Standards National Bureau of Standards Boulder, Colorado 80303 Sponsored by National Bureau of Standards and Office of Naval Research Arlington, Virginia 22217 U.S. DEPARTMENT OF COMMERCE, Juanita M. Kreps, Secretary Dr. Sidney Harman, Under Secretary Jordan J. Baruch, Assistant Secretary for Science and Technology NATIONAL BUREAU OF STANDARDS, Ernest Ambler, Director Issued April 1978 Library of Congress Catalog Card Number: 78-606017 National Bureau of Standards Special Publication 508 Nat. Bur. Stand. (U.S.) Spec. Publ. 508, 238 pages (Apr. 1978) CODEN. XNBSAV U.S. GOVERNMENT PRINTING OFFICE WASHINGTON: 1978 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20434 Stock No 003-003-01910-1 Price $4.25 (Add 25 percent additional for other than U.S. mailing). ABSTRACT This document contains the proceedings of a meeting of specialists in small superconducting devices and in small cryogenic refrigerators. Industry, Government, and academia were represented at the meeting held at the National Bureau of Standards (NBS) on October 3 and 4, 1977« The purpose of the meeting was to define the refrigerator requirements for small superconducting devices and to determine if small cryogenic refrigerators that are produced in relatively large quantities can be adapted or developed to replace liquid helium as the cooling medium for the superconducting devices. -
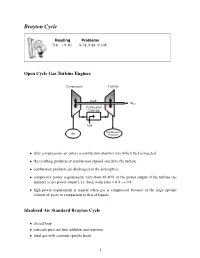
Brayton Cycle
Brayton Cycle Reading Problems 9-8 → 9-10 9-78, 9-84, 9-108 Open Cycle Gas Turbine Engines • after compression, air enters a combustion chamber into which fuel is injected • the resulting products of combustion expand and drive the turbine • combustion products are discharged to the atmosphere • compressor power requirements vary from 40-80% of the power output of the turbine (re- mainder is net power output), i.e. back work ratio = 0.4 → 0.8 • high power requirement is typical when gas is compressed because of the large specific volume of gases in comparison to that of liquids Idealized Air Standard Brayton Cycle • closed loop • constant pressure heat addition and rejection • ideal gas with constant specific heats 1 Brayton Cycle Efficiency The Brayton cycle efficiency can be written as (1−k)/k η =1− (rp) wherewedefine the pressure ratio as: P2 P3 rp = = P1 P4 2 Maximum Pressure Ratio Given that the maximum and minimum temperature can be prescribed for the Brayton cycle, a change in the pressure ratio can result in a change in the work output from the cycle. The maximum temperature in the cycle (T3) is limited by metallurgical conditions because the turbine blades cannot sustain temperatures above 1300 K. Higher temperatures (up to 1600 K can be obtained with ceramic turbine blades). The minimum temperature is set by the air temperature at the inlet to the engine. 3 Brayton Cycle with Reheat • T3 is limited due to metallurgical constraints • excess air is extracted and fed into a second stage combustor and turbine • turbine outlet temperature -

Liquid-Flooded Ericsson Cycle Cooler: Part 1 – Thermodynamic Analysis Jason Hugenroth Purdue University
Purdue University Purdue e-Pubs International Refrigeration and Air Conditioning School of Mechanical Engineering Conference 2006 Liquid-Flooded Ericsson Cycle Cooler: Part 1 – Thermodynamic Analysis Jason Hugenroth Purdue University James Braun Purdue University Eckhard Groll Purdue University Galen King Purdue University Follow this and additional works at: http://docs.lib.purdue.edu/iracc Hugenroth, Jason; Braun, James; Groll, Eckhard; and King, Galen, "Liquid-Flooded Ericsson Cycle Cooler: Part 1 – Thermodynamic Analysis" (2006). International Refrigeration and Air Conditioning Conference. Paper 823. http://docs.lib.purdue.edu/iracc/823 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Complete proceedings may be acquired in print and on CD-ROM directly from the Ray W. Herrick Laboratories at https://engineering.purdue.edu/ Herrick/Events/orderlit.html R168, Page 1 LIQUID-FLOODED ERICSSON CYCLE COOLER: PART 1- THERMODYNAMIC ANALYSIS Jason HUGENROTH1,*, Jim BRAUN2, Eckhard GROLL3, Galen KING4 Purdue University, Mechanical Engineering, Herrick Laboratories West Lafayette, Indiana, USA [email protected] [email protected] [email protected] [email protected] *Corresponding author ABSTRACT A novel implementation of a gas Ericsson cycle heat pump is presented. The concept uses liquid flooding of the compressor and expander to approach isothermal compression and expansion processes. A thermodynamic analysis of the cycle was performed using an EES-based computer model. In the ideal case with reversible components, the coefficient of performance (COP) of the cooler approaches the Carnot COP as the liquid flooding is increased. However, in the nonideal case when the rotating machinery operates irreversibly, there is an optimal liquid flooding rate and pressure ratio that produces the best performance.