Physiology of Heart Unit-4 (ZOOA-CC4-9-TH)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Abnormally Enlarged Singular Thebesian Vein in Right Atrium
Open Access Case Report DOI: 10.7759/cureus.16300 Abnormally Enlarged Singular Thebesian Vein in Right Atrium Dilip Kumar 1 , Amit Malviya 2 , Bishwajeet Saikia 3 , Bhupen Barman 4 , Anunay Gupta 5 1. Cardiology, Medica Institute of Cardiac Sciences, Kolkata, IND 2. Cardiology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 3. Anatomy, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 4. Internal Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 5. Cardiology, Vardhman Mahavir Medical College (VMMC) and Safdarjung Hospital, New Delhi, IND Corresponding author: Amit Malviya, [email protected] Abstract Thebesian veins in the heart are subendocardial venoluminal channels and are usually less than 0.5 mm in diameter. The system of TV either opens a venous (venoluminal) or an arterial (arterioluminal) channel directly into the lumen of the cardiac chambers or via some intervening spaces (venosinusoidal/ arteriosinusoidal) termed as sinusoids. Enlarged thebesian veins are reported in patients with congenital heart disease and usually, multiple veins are enlarged. Very few reports of such abnormal enlargement are there in the absence of congenital heart disease, but in all such cases, they are multiple and in association with coronary artery microfistule. We report a very rare case of a singular thebesian vein in the right atrium, which was abnormally enlarged. It is important to recognize because it can be confused with other cardiac structures like coronary sinus during diagnostic or therapeutic catheterization and can lead to cardiac injury and complications if it is attempted to cannulate it or pass the guidewires. -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -

Cardiology Self Learning Package
Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Module 1: Anatomy and Physiology of the Heart Heart. Page 1 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) CONTENT Introduction…………………………………………………………………………………Page 3 How to use the ECG Self Learning package………………………………………….Page 4 Overview of the Heart…………………………………………………...…………..…….Page 5 Location, Size and Shape of the Heart…………………………………………………Page 5 The Chambers of the Heart…………….………………………………………..……….Page 7 The Circulation System……………………………………….………………..…………Page 8 The Heart Valve Anatomy………………………….…………………………..…………Page 9 Coronary Arteries…………………………………………….……………………..……Page 10 Coronary Veins…………………………………………………………………..……….Page 11 Cardiac Muscle Tissue……………………………………………………………..……Page 12 The Conduction System………………………………………………………………...Page 13 Cardiac Cycle……………………………………………………………………………..Page 15 References…………………………………………………………………………………Page 18 Module Questions………………………………………………………………………..Page 19 Module Evaluation Form………………………………………………………………..Page 22 [Module 1: Anatomy and Physiology of the Heart Page 2 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) INTRODUCTION Welcome to Module 1: Anatomy and Physiology of the Heart. This self leaning package is designed to as tool to assist nurse in understanding the hearts structure and how the heart works. The goal of this module is to review: Location , size and shape of the heart The chambers of the heart The circulation system of the heart The heart’s valve anatomy Coronary arteries and veins Cardiac muscle tissue The conduction system The cardiac cycle This module will form the foundation of your cardiac knowledge and enable you to understand workings of the heart that will assist you in completing other modules. Learning outcomes form this module are: To state the position of the heart, the size and shape. -

Unit 1 Lecture 2
Unit 1 Lecture 2 Unit 1 Lecture 2 THE HEART Anatomy of the Heart The heart rests on the diaphragm in a space called the mediastinum. It weighs @ 300 grams and is about as big as a clenched fist. The pointed end is called the apex and the opposite end is called the base but is really the top of the heart. The bulk of the heart tissue is made up of the left ventricle. A pericardium (a 3-layered bag) surrounds the heart and composed of fibrous pericardium (that provides protection to the heart, prevents overstretching, and anchors the heart in the mediastinum), a serous pericardium which is composed of two layers (the parietal layer or outer layer that is fused to the fibrous pericardium and the visceral layer or the inner layer that adheres to heart muscle). The visceral pericardium is also called the epicardium. Pericardial fluid is found in the pericardial cavity, which is located between the two layers, helps lubricate and reduces friction between membranes as the heart moves. The heart wall is composed of three layers: the epicardium (see visceral pericardium above), the myocardium which is cardiac muscle tissue and the bulk of mass in the heart, and endocardium or the innermost layer of the heart. This lining is continuous through all of the blood vessels except for the capillaries. The Heart Chambers and Valves Internally the heart is divided into four chambers. The two upper chambers are the right and left atria. Each has an appendage (auricle) whose function is to increase the volume of the atria. -

REVIEW ARTICLE Anatomy and Physiology of Coronary Blood Flow
REVIEW ARTICLE Anatomy and physiology of coronary blood flow Heinrich R. Schelbert, MD, PhD INTRODUCTION hypertrophic cardiomyopathy, or coronary artery disease, resting myocardial blood flows frequently are similar to Regional myocardial blood flow can now be mea- those in normal individuals.17-19 It is the response of sured noninvasively in units of milliliters blood per myocardial blood flow to specifically-targeted pharma- minute per gram myocardium. These noninvasive mea- cological or physiological interventions that can uncover surements are not confined to a specific imaging the presence of functional or structural disease-related modality but are available with MRI, CT, and PET, alterations of the coronary circulation. This then under- although, thus far, most investigations of the coronary scores the need for closely examining these targets and circulation in humans have employed PET flow mea- how they relate to anatomical and functional determi- surements. Flow estimates with these different imaging nants of coronary blood flow and, by inference, to modalities were found in animal experiments to correlate myocardial blood flow and their alterations in cardio- well with invasive flow estimates by the arterial blood vascular disease. Local and systemic mechanisms sampling-microsphere technique widely considered as regulate the complex interactions between flow and the ‘‘gold standard’’ of flow measurements.1-11 In these anatomy in order to meet the heart’s energy needs. comparison studies, noninvasively-derived estimates A comprehensive description of the coronary circulatory corresponded linearly with invasively-measured myo- function and its control exceeds the scope of this review cardial blood flows over a wide flow range, i.e., from as so that the interested reader is referred to detailed reviews low as 0.3 mL/minute/g to as high as 5-6 mL/minute/g. -

Unit 1 Anatomy of the Heart
UNIT 1 ANATOMY OF THE HEART Structure 1.0 Objectives 1.1 Introduction 1.2 Chambers of Heart 1.3 Orifices of Heart 1.4 The Conducting System of the Heart 1.5 Blood Supply of the Heart 1.6 Surface Markings of the Heart 1.7 Let Us Sum Up 1.8 Answers to Check Your Progress 1.0 OBJECTIVES After reading this unit, you should be able to: • understand the proper position of a heart inside the thorax; • know the various anatomical structures of various chambers of heart and various valves of heart; • know the arterial supply, venous drainage and lymphatic drainage of heart; and • describe the surface marking of heart. 1.1 INTRODUCTION The human heart is a cone-shaped, four-chambered muscular pump located in the mediastinal cavity of the thorax between the lungs and beneath the sternum, designed to ensure the circulation through the tissues of the body. The cone-shaped heart lies on its side on the diaphragm, with its base (the widest part) upward and leaning toward the right shoulder, and its apex pointing down and to the left. Structurally and functionally it consists of two halves–right and left. The right heart circulates blood only through the lungs for the purpose of pulmonary circulation. The left heart sends blood to tissues of entire body/systemic circulation. The heart is contained in a sac called the pericardium. The four chambers are right and left atria and right and left ventricles. The heart lies obliquely across the thorax and the right side is turned to face the front. -

Chapter Twenty
Chapter 22 Outline • Overview of the Cardiovascular System • Anatomy of the Heart • Coronary Circulation • How the Heart Beats: Electrical Properties of Cardiac Tissue • Innervation of the Heart • Tying It All Together: The Cardiac Cycle • Aging and the Heart • Development of the Heart Overview of the Cardiovascular System • The heart propels blood to and from most body tissues via two basic types of blood vessels called ______ and ______. • Arteries are defined as blood vessels that carry blood away from the heart. • Veins are defined as blood vessels that carry blood back to the heart. • The arteries and veins entering and leaving the heart are called ______ vessels. General Characteristics and Functions of the Heart • Blood flow through the heart is ______ because of four valves within the heart. • The heart is functionally two side-by-side pumps that work at the same rate and pump the same volume of blood. – One pump directs blood to the lungs. – One pump directs blood to most body tissues. General Characteristics and Functions of the Heart • The heart generates ______ pressure through alternate cycles of the heart wall’s contraction and relaxation. • Blood pressure is the force of the blood pushing against the inside walls of blood vessels. • A minimum blood pressure is essential to circulate blood throughout the body. Pulmonary and Systemic Circulations The cardiovascular system consists of two circulations: 1. ______—right side of the heart and the pulmonary arteries and veins; conveys blood to the lungs and back to the left side of the heart 2. ______—left side of the heart and arteries and veins; conveys blood to most body tissues and back to the right side of the heart Cardiovascular System Figure 22.1 Position of the Heart • Slightly left of midline deep to the sternum in a compartment of the thorax known as the mediastinum Figure 22.2 Position of the Heart • During development, the heart rotates such that the right side or right border (primarily formed by the right atrium and ventricle) is located more anteriorly. -

22. Heart.Pdf
CARDIOVASCULAR SYSTEM OUTLINE 22.1 Overview of the Cardiovascular System 657 22.1a Pulmonary and Systemic Circulations 657 22.1b Position of the Heart 658 22 22.1c Characteristics of the Pericardium 659 22.2 Anatomy of the Heart 660 22.2a Heart Wall Structure 660 22.2b External Heart Anatomy 660 Heart 22.2c Internal Heart Anatomy: Chambers and Valves 660 22.3 Coronary Circulation 666 22.4 How the Heart Beats: Electrical Properties of Cardiac Tissue 668 22.4a Characteristics of Cardiac Muscle Tissue 668 22.4b Contraction of Heart Muscle 669 22.4c The Heart’s Conducting System 670 22.5 Innervation of the Heart 672 22.6 Tying It All Together: The Cardiac Cycle 673 22.6a Steps in the Cardiac Cycle 673 22.6b Summary of Blood Flow During the Cardiac Cycle 673 22.7 Aging and the Heart 677 22.8 Development of the Heart 677 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch22_656-682.indd 656 2/14/11 4:29 PM Chapter Twenty-Two Heart 657 n chapter 21, we discovered the importance of blood and the which carry blood back to the heart. The differences between I myriad of substances it carries. To maintain homeostasis, blood these types of vessels are discussed in chapter 23. Most arteries must circulate continuously throughout the body. The continual carry blood high in oxygen (except for the pulmonary arteries, pumping action of the heart is essential for maintaining blood as explained later), while most veins carry blood low in oxygen circulation. If the heart fails to pump adequate volumes of blood, (except for the pulmonary veins). -
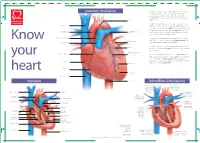
Structure Coronary Circulation
B C M Y X CMY B5 C5 M5 Y5 X5 B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y Prinect/FOGRA 5 Dipco 2.1 Format 105 © 2004 FOGRA/Heidelberger Druckmaschinen AG B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y X B C M Y X B C M Y X B5 C5 M5 Y5 X5 CMY B C M Y X B C M Y X CMY B5 C5 M5 Y5 X5 B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y Prinect/FOGRA 5 Dipco 2.1 Format 105 © 2004 FOGRA/Heidelberger Druckmaschinen AG B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y X B C M Y X B C M Y X B5 C5 M5 Y5 X5 CMY B C M Y X 5 5 5 5 5 5 5 5 5 5 4 4 4 4 4 4 4 4 4 4 3 3 3 3 3 3 3 3 3 3 2 2 2 2 2 2 2 2 2 2 1 1 1 1 1 1 1 1 1 1 1234567891011121314151617181920212223242526272829303132 coronary circulation The heart is a continuous pump which distributes blood to every part of the body. Blood carries essential oxygen to the tissues and carries away unwanted carbon dioxide and other waste products. -

G06, G12, G13 (1000263, 1000268, 1000269) 2 Latin
…going one step further G06 1000263 G13 1000269 G12 1000268 G06, G12, G13 (1000263, 1000268, 1000269) 2 Latin G12, G13: A Apex cordis B Septum interventriculare, pars muscularis I Atrium dextrum Ia Auricula dextra II Atrium sinistrum IIb Auricula sinistra III Ventriculus dexter IV Ventriculus sinister 1 V. cava superior 1a V. brachiocephalica sinistra 2 V. cava inferior 3 Valva atrioventricularis dextra (Valva tricuspidalis) 3a Mm. papillares 3b Valva trunci pulmonalis 4 Truncus pulmonalis 4a A. pulmonalis sinistra 4b A. pulmonalis dextra 5 Vv. pulmonales 6 Valva atrioventricularis sinistra (Valva mitralis) 6c Mm. papillares 6d Valva aortae 7 Pars ascendens aortae ® 7a Arcus aortae 7b Truncus brachiocephalicus 7c A. carotis communis sinistra 7d A. subclavia sinistra 8 A. coronaria dextra 8a R. interventricularis posterior a. coronariae dextrae 8b R. posterolateralis dexter a. coronariae dextrae 9a R. interventricularis anterior a. coronariae sinistrae 9b R. circumflexus a. coronariae sinistrae 9c R. lateralis a. coronariae sinistrae 10 Sinus coronarius 10a V. cardiaca magna 10b V. cardiaca parva 10c V. cardiaca media (V. interventricularis posterior) 10d Vv. ventriculi sinisteri posteriores 11 Sulcus coronarius 12 Sulcus interventricularis anterior 13 Sulcus interventricularis posterior G13: 14 Trachea 15 Oesophagus 3 Heart, 2-times life size English G12, G13: A Apex of heart B Muscular part of interventricular septum I Right atrium Ia Right auricle II Left atrium IIb Left auricle III Right ventricle IV Left ventricle 1 Superior vena -

Anatomic Variations of Coronary Arteries: Origins, Branching Patterns, and Abnormalities Natatcha Khwansang, Vilai Chentanez*
DOI 10.1515/abm-2019-0010 — Asian Biomed (Res Rev News) 2018; 12(3 Anat issue Pt 1):117–123 Open access Brief communication (original) Anatomic variations of coronary arteries: origins, branching patterns, and abnormalities Natatcha Khwansang, Vilai Chentanez* Abstract Background: Anatomic variations in orifices, courses, branching patterns, and abnormalities of coronary arteries could affect blood supply, hemodynamic characteristics, and clinical symptoms, and could be a risk of atherosclerosis. Objectives: To investigate the location and number of both coronary orifices in the aortic cusps, branching patterns of left main trunk, dominant pattern of posterior interventricular artery (PIA), prevalence of right posterior diagonal artery (RPDA), myocardial bridge, and other abnormalities. Methods: We dissected 95 heart specimens from cadavers of Thai donors without the history of surgery, and the dominant patterns, location and number of orifices in the aortic cusps, branching patterns, origin and number of conal arteries, and occurrence of RPDA were determined. Results: Dual aortic origin of the coronary orifice was the most common condition. Anomalous 2 orifices in the left aortic cusp were found in one specimen in which the right coronary artery (RCA) arose from aortic cusp and had an interarterial course. Right dominance and trifurcated form of left main trunk were found more frequently. Most frequently 2 conal arteries were found. RPDA was found in 45% and mostly originated from RCA. The prevalence of myocardial bridge was 62% and located mostly on the anterior interventricular artery (AIA). Conclusions: The prevalence of right dominance, RPDA, the atypical origin of RCA from the left sinus, and the prevalence of myocardial bridges was more frequent than reported by others, whereas the dual aortic origin from both cusps and the prevalence of bifurcated left main trunk was less frequent.