Severe Rhabdomyolysis and Acute Renal Failure Secondary to Concomitant Use of Simvastatin, Amiodarone, and Atazanavir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

The Differential Effects of Statins on the Metastatic Behaviour of Prostate Cancer
British Journal of Cancer (2012) 106, 1689–1696 & 2012 Cancer Research UK All rights reserved 0007 – 0920/12 www.bjcancer.com The differential effects of statins on the metastatic behaviour of prostate cancer *,1 1 1 2,3 2,3 2,4 1,2,4 M Brown , C Hart , T Tawadros , V Ramani , V Sangar , M Lau and N Clarke 1 Genito Urinary Cancer Research Group, University of Manchester, Paterson Institute for Cancer Research, Manchester Academic Health Science Centre, 2 The Christie NHS Foundation Trust, Wilmslow Road, Withington, Manchester M20 4BX, UK; Department of Urology, The Christie NHS Foundation 3 Trust, Wilmslow Road, Manchester M20 4BX, UK; Department of Urology, University Hospital of South Manchester NHS Trust, Manchester M23 9LT, 4 UK; Department of Urology, Salford Royal NHS Foundation Trust, Stott Lane, Manchester M6 8HD, UK BACKGROUND: Although statins do not affect the incidence of prostate cancer (CaP), usage reduces the risk of clinical progression and mortality. Although statins are known to downregulate the mevalonate pathway, the mechanism by which statins reduce CaP progression is unknown. METHODS: Bone marrow stroma (BMS) was isolated with ethical approval from consenting patients undergoing surgery for non- malignant disease. PC-3 binding, invasion and colony formation within BMS was assessed by standardised in vitro co-culture assays in the presence of different statins. RESULTS: Statins act directly on PC-3 cells with atorvastatin, mevastatin, simvastatin (1 mM) and rosuvastatin (5 mM), but not pravastatin, significantly reducing invasion towards BMS by an average of 66.68% (range 53.93–77.04%; Po0.05) and significantly reducing both 2 2 number (76.2±8.29 vs 122.9±2.48; P ¼ 0.0055) and size (0.2±0.0058 mm vs 0.27±0.012 mm ; P ¼ 0.0019) of colonies formed within BMS. -

What Precautions Should We Use with Statins for Women of Childbearing
CLINICAL INQUIRIES What precautions should we use with statins for women of childbearing age? Chaitany Patel, MD, Lisa Edgerton, PharmD New Hanover Regional Medical Center, Wilmington, North Carolina Donna Flake, MSLS, MSAS Coastal Area Health Education Center, Wilmington, NC EVIDENCE- BASED ANSWER Statins are contraindicated for women who are on its low tissue-penetration properties. pregnant or breastfeeding. Data evaluating statin Cholesterol-lowering with simvastatin 40 mg/d did use for women of childbearing age is limited; how- not disrupt menstrual cycles or effect luteal phase ever, they may be used cautiously with adequate duration (strength of recommendation: C). contraception. Pravastatin may be preferred based CLINICAL COMMENTARY Use statins only as a last resort Before reading this review, I had not been for women of childbearing age ® Dowdenaware Health of the serious Media effects of statin medications I try to follow the USPSTF recommendations and on the developing fetus. In conversations with not screen women aged <45 years without coro- my colleagues, I found that the adverse effects nary artery disease riskCopyright factors for Fhyperlipidemia.or personalof usestatins onlyduring pregnancy are not readily When a woman of any age needs treatment, my known. Such information needs to be more first-line therapy is lifestyle modification. Given the widely disseminated. risks of statin drugs to the developing fetus, Ariel Smits, MD women with childbearing potential should give Department of Family Medicine, Oregon Health & Science fully informed consent and be offered reliable University, Portland contraception before stating statin therapy. I Evidence summary anal, cardiac, tracheal, esophageal, renal, Hydroxymethyl glutaryl coenzyme A and limb deficiency (VACTERL associa- (HMG CoA) reductase inhibitors, com- tion), intrauterine growth retardation monly called statins, have been on the (IUGR), and demise in fetuses exposed market since the late 1980s. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -

Prevalence of Drug Interactions in Hospitalized Elderly Patients: a Systematic Review
Supplementary material Eur J Hosp Pharm Prevalence of drug interactions in hospitalized elderly patients: a systematic review Luciana Mello de Oliveira 1,2; Juliana do Amaral Carneiro Diel1; Alessandra Nunes3; Tatiane da Silva Dal Pizzol 1,2,3 1Programa de Pós-Graduação em Epidemiologia, Faculdade de Medicina, Universidade Federal do Rio Grande do Sul. 2Programa de Pós-Graduação em Assistência Farmacêutica, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul. 3Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul. Corresponding author: Luciana Mello de Oliveira – [email protected] and Tatiane da Silva Dal Pizzol - [email protected] Supplementary Table 3: Number of patients with interaction, number of DDI per patient with at least one DDI, drugs or drug classes mostly involved with DDI and drug combinations mostly involved with DDI. In cases which prevalence were described, we reported the three drugs mostly involved with drug interactions or the three drug combinations (or drug classes) mostly involved with DDI. ACE: angiotensin-converting enzyme. NA: not available. NSAID: non-steroidal anti-inflammatory drugs. PPI: proton-pump inhibitors. # of patients with # of DDI per patient with First autor interactions interaction Drugs or drug classes mostly involved with DDI Drug combinations mostly involved with DDI Barak-Tsarfir O, et al (61) Unclear: around 56 patients NA NA NA Warfarin; digitoxin; prednisolone antithrombotic agents; non-steroidal anti- 70 (evaluated only serious or inflammatory agents; angiotensin converting enzyme Blix HS, et al (29) contraindicated DDI) NA inhibitors N/A Serious: chlorpromazine + promethazine; chlorpromazine + haloperidol; haloperidol + promethazine; diazepam + phenobarbital; risperidone + haloperidol; carbamazepine + ketoconazole; carbamazepine + chlorpromazine; haloperidol + ketoconazole; chlorpromazine + ketoconazole; chlorpromazine + sodium phosphate. -

An Update on the Efficacy of Anti-Inflammatory Agents for Patients with Schizophrenia: Cambridge.Org/Psm a Meta-Analysis
Psychological Medicine An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: cambridge.org/psm a meta-analysis 1,2 2,3,4 2,5 6 Review Article N. Çakici , N. J. M. van Beveren , G. Judge-Hundal , M. M. Koola and I. E. C. Sommer5 Cite this article: Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC 1Department of Psychiatry and Amsterdam Neuroscience, Academic Medical Center, Meibergdreef 9, 1105 AZ (2019). An update on the efficacy of anti- Amsterdam, the Netherlands; 2Antes Center for Mental Health Care, Albrandswaardsedijk 74, 3172 AA, Poortugaal, inflammatory agents for patients with 3 schizophrenia: a meta-analysis. Psychological the Netherlands; Department of Psychiatry, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD 4 Medicine 49, 2307–2319. https://doi.org/ Rotterdam, the Netherlands; Department of Neuroscience, Erasmus Medical Center, Doctor Molewaterplein 40, 5 10.1017/S0033291719001995 3015 GD Rotterdam, the Netherlands; Department of Psychiatry and Biomedical Sciences of Cells and Systems, University Medical Center Groningen, Deusinglaan 2, 9713AW Groningen, the Netherlands and 6Department of Received: 13 February 2019 Psychiatry and Behavioral Sciences, George Washington University School of Medicine and Health Sciences, 2300I Revised: 4 July 2019 St NW, Washington, DC 20052, USA Accepted: 16 July 2019 First published online: 23 August 2019 Abstract Key words: Background. Accumulating evidence shows that a propensity towards a pro-inflammatory Add-on antipsychotic therapy; estrogens; fatty acids; minocycline; N-acetylcysteine status in the brain plays an important role in schizophrenia. Anti-inflammatory drugs might compensate this propensity. This study provides an update regarding the efficacy of Author for correspondence: agents with some anti-inflammatory actions for schizophrenia symptoms tested in rando- N. -
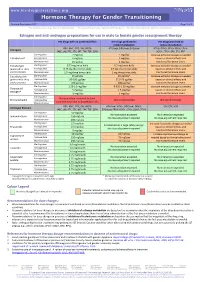
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -
Simvastatin 80Mg Tablets
Package leaflet: Information for the patient Simvastatin 80mg Tablets Read all of this leaflet carefully before you • if you are due to have an operation. You may need start taking this medicine because it contains to stop taking Simvastatin tablets for a short time important information for you. • if you are Asian, because a different dose may be • Keep this leaflet. You may need to read it again. applicable to you • If you have any further questions, ask your • if you are taking or have taken in the last 7 days doctor or pharmacist. a medicine called fusidic acid (a medicine for bacterial infection) orally or by injection. The • This medicine has been prescribed for you only. combination of fusidic acid and Simvastatin can Do not pass it on to others. It may harm them, lead to serious muscle problems (rhabdomyolysis). even if their signs of illness are the same as yours. Your doctor should do a blood test before you start • If you get any side effects, talk to your doctor taking Simvastatin and if you have any symptoms of or pharmacist. This includes any possible side liver problems while you take Simvastatin. This is to effects not listed in this leaflet. See section 4. check how well your liver is working. What is in this leaflet Your doctor may also want you to have blood tests to check how well your liver is working after you 1 What Simvastatin is and what it is used start taking Simvastatin. for 2 What you need to know before you take While you are on this medicine your doctor will Simvastatin monitor you closely if you have diabetes or are at risk of developing diabetes. -

Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Details Generic Name Indinavir Trade Name Crixivan® Class Protease Inhibitor Molecular Weight 711.88 (as sulphate) Structure HO OH N N H H SO N N 2 4 O HN O H3C CH3 H3C Summary of Key Pharmacokinetic Parameters Plasma half life 1.8 ± 0.4 h Cmax 8.97 ± 2.87 µg/ml (800 mg every 8 hours) Cmin 0.146 µg/ml (800 mg every 8 hours) AUC 21.82 ± 8.11 µg/ml.h (800 mg every 8 hours) Bioavailability Approx 65% Absorption Administration of indinavir with a meal high in calories, fat, and protein resulted in a blunted and reduced absorption with an approximate 80 % reduction in AUC and an 86 % reduction in Cmax. Administration with light meals (e.g., dry toast with jam or fruit conserve, apple juice, and coffee with skimmed or fat–free milk and sugar or corn flakes, skimmed or fat–free milk and sugar) resulted in plasma concentrations comparable to the corresponding fasted values. Protein Binding ~60% Volume of Distribution ~1.74 L/kg [1] CSF:Plasma ratio 0.14 [2] Semen:Plasma ratio 1.9 [2] Renal Clearance <20% as unchanged drug Renal Impairment Safety in patients with impaired renal function has not been studied; less than 20% of indinavir is excreted in the urine as unchanged drug or metabolites. Hepatic Impairment Safety and efficacy of indinavir has not been established in patients with significant underlying liver disorders and should be used with caution. -

Drug-Drug Interaction Between Protease Inhibitors and Statins and Proton Pump Inhibitors
Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Item Type text; Electronic Report Authors Orido, Charles; McKinnon, Samantha Publisher The University of Arizona. Rights Copyright © is held by the author. Download date 01/10/2021 01:48:07 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/636245 Group 47 :Orido/Samantha 1 Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Course Title: PhPr 862 Date: April 3, 2019 Faculty Advisor: Dr. Dan Malone Students: Charles Orido, Samantha McKinnon Pharm.D. Candidates, Class of 2019 Group 47 :Orido/Samantha 2 Objective The purpose of this article is to provide a systematic review of the pharmacokinetic and clinical data on drug-drug interactions between protease inhibitors (PIs) and statins, atazanavir and proton pump inhibitors (PPIs)and their clinical relevance. Methods A literature search was performed using Medline, EMBASE and google scholar, abstracts from 1970 to 2019 of major conferences were searched and FDA drug information package inserts of the manufacturer of every currently available PI was looked at. All data was summarized and verified by at least two investigators. Results A total of 246 references were identified, 8 of which were studies of pharmacokinetic and pharmacodynamics interactions between simvastatin, lovastatin and protease inhibitors and an additional 7 articles that provided pharmacokinetic of proton pump inhibitors and Atazanavir. Conclusions Protease inhibitors increases the AUC and Cmax of simvastatin by approximately 500% and 517% respectively. Therefore, simvastatin and Lovastatin are not recommended for a co-administration with a protease inhibitor. -

Crixivan (Indinavir Sulfate)
XXXXXXX CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN* (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, and 400 mg of indinavir (corresponding to 125, 250, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin and titanium dioxide. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2 (phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4 • H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. MICROBIOLOGY Mechanism of Action: HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Antiretroviral Activity In Vitro: The in vitro activity of indinavir was assessed in cell lines of lymphoblastic and monocytic origin and in peripheral blood lymphocytes. HIV-1 variants used to infect the different cell types include laboratory-adapted variants, primary clinical isolates and clinical isolates resistant to nucleoside analogue and nonnucleoside inhibitors of the HIV-1 reverse transcriptase. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017.