Ongoing Living Update of Potential COVID-19 Therapeutics: Summary of Rapid Systematic Reviews
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19) Pandemic on National Antimicrobial Consumption in Jordan
antibiotics Article An Assessment of the Impact of Coronavirus Disease (COVID-19) Pandemic on National Antimicrobial Consumption in Jordan Sayer Al-Azzam 1, Nizar Mahmoud Mhaidat 1, Hayaa A. Banat 2, Mohammad Alfaour 2, Dana Samih Ahmad 2, Arno Muller 3, Adi Al-Nuseirat 4 , Elizabeth A. Lattyak 5, Barbara R. Conway 6,7 and Mamoon A. Aldeyab 6,* 1 Clinical Pharmacy Department, Jordan University of Science and Technology, Irbid 22110, Jordan; [email protected] (S.A.-A.); [email protected] (N.M.M.) 2 Jordan Food and Drug Administration (JFDA), Amman 11181, Jordan; [email protected] (H.A.B.); [email protected] (M.A.); [email protected] (D.S.A.) 3 Antimicrobial Resistance Division, World Health Organization, Avenue Appia 20, 1211 Geneva, Switzerland; [email protected] 4 World Health Organization Regional Office for the Eastern Mediterranean, Cairo 11371, Egypt; [email protected] 5 Scientific Computing Associates Corp., River Forest, IL 60305, USA; [email protected] 6 Department of Pharmacy, School of Applied Sciences, University of Huddersfield, Huddersfield HD1 3DH, UK; [email protected] 7 Institute of Skin Integrity and Infection Prevention, University of Huddersfield, Huddersfield HD1 3DH, UK * Correspondence: [email protected] Citation: Al-Azzam, S.; Mhaidat, N.M.; Banat, H.A.; Alfaour, M.; Abstract: Coronavirus disease 2019 (COVID-19) has overlapping clinical characteristics with bacterial Ahmad, D.S.; Muller, A.; Al-Nuseirat, respiratory tract infection, leading to the prescription of potentially unnecessary antibiotics. This A.; Lattyak, E.A.; Conway, B.R.; study aimed at measuring changes and patterns of national antimicrobial use for one year preceding Aldeyab, M.A. -

(MGH) COVID-19 Treatment Guidance
Version 8.0 4/28/2021 10:00AM © Copyright 2020 The General Hospital Corporation. All Rights Reserved. Massachusetts General Hospital (MGH) COVID-19 Treatment Guidance This document was prepared (in March, 2020-April, 2021) by and for MGH medical professionals (a.k.a. clinicians, care givers) and is being made available publicly for informational purposes only, in the context of a public health emergency related to COVID-19 (a.k.a. the coronavirus) and in connection with the state of emergency declared by the Governor of the Commonwealth of Massachusetts and the President of the United States. It is neither an attempt to substitute for the practice of medicine nor as a substitute for the provision of any medical professional services. Furthermore, the content is not meant to be complete, exhaustive, or a substitute for medical professional advice, diagnosis, or treatment. The information herein should be adapted to each specific patient based on the treating medical professional’s independent professional judgment and consideration of the patient’s needs, the resources available at the location from where the medical professional services are being provided (e.g., healthcare institution, ambulatory clinic, physician’s office, etc.), and any other unique circumstances. This information should not be used to replace, substitute for, or overrule a qualified medical professional’s judgment. This website may contain third party materials and/or links to third party materials and third party websites for your information and convenience. Partners is not responsible for the availability, accuracy, or content of any of those third party materials or websites nor does it endorse them. -

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -
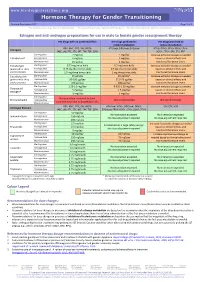
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -

Progress in the Development of Potential Therapeutics and Vaccines Against COVID-19 Pandemic
Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423) Volume 5 Issue 7 July 2021 Review Article Progress in the Development of Potential Therapeutics and Vaccines against COVID-19 Pandemic Abhishek Kumar Yadav, Shubham Kumar and Vikramdeep Monga* Received: May 02, 2021 Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, Punjab, Published: June 09, 2021 India © All rights are reserved by Vikramdeep *Corresponding Author: Vikramdeep Monga, Department of Pharmaceutical Monga., et al. Chemistry, ISF College of Pharmacy, Moga, Punjab, India. Abstract Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 or coronavirus disease 2019 and the same has been declared as a global pandemic by WHO which marked the third introduction of a virulent coronavirus into human society. This a threat to human life worldwide. Considerable efforts have been made for developing effective and safe drugs and vaccines against is a highly pathogenic human coronavirus in which pneumonia of unknown origin was identified in China in December 2019 and is SARS-CoV-2. The current situation and progress in the development of various therapeutic candidates including vaccines in preclini- cal and clinical studies have been described in the manuscript. Until now, many people have been infected with this lethal virus, and a lot of people have died from this COVID-19. This viral disease spreads by coming in contact with an infected person. Understand- ing of SARS-CoV-2 is growing in relation to its epidemiology, virology, and clinical management strategies. Till date, very few drugs or vaccines have been developed or approved for the treatment of this deadly disease of COVID-19 and many candidates are under the clinical development pipeline. -

Impact of SARS-Cov-2 Variant on the Severity of Maternal Infection and Perinatal Outcomes: Data from the UK Obstetric Surveillan
medRxiv preprint doi: https://doi.org/10.1101/2021.07.22.21261000; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . Impact of SARS-CoV-2 variant on the severity of maternal infection and perinatal outcomes: Data from the UK Obstetric Surveillance System national cohort. Nicola Vousden PhD1, Rema Ramakrishnan PhD1, Kathryn Bunch MA1, Edward Morris MD2, Nigel Simpson MBBS3, Christopher Gale PhD4, Patrick O’Brien MBBCh,2,5, Maria Quigley MSc1, Peter Brocklehurst MSc6, Jennifer J Kurinczuk MD1, Marian Knight DPhil1 1National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, UK 2Royal College of Obstetricians and Gynaecologists, London, UK 3Department of Women's and Children's Health, School of Medicine, University of Leeds, UK 4Neonatal Medicine, School of Public Health, Faculty of Medicine, Imperial College London, London, UK 5Institute for Women’s Health, University College London, London, UK 6Birmingham Clinical Trials Unit, Institute of Applied Health Research, University of Birmingham, UK *Corresponding author: Marian Knight National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, UK 01865 289700 [email protected] NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. 1 medRxiv preprint doi: https://doi.org/10.1101/2021.07.22.21261000; this version posted July 25, 2021. -

Determining Ribavirin's Mechanism of Action Against Lassa Virus Infection
www.nature.com/scientificreports OPEN Determining Ribavirin’s mechanism of action against Lassa virus infection Received: 28 March 2017 Paola Carrillo-Bustamante1, Thi Huyen Tram Nguyen2,3, Lisa Oestereich4,5, Stephan Accepted: 4 August 2017 Günther4,5, Jeremie Guedj 2,3 & Frederik Graw1 Published: xx xx xxxx Ribavirin is a broad spectrum antiviral which inhibits Lassa virus (LASV) replication in vitro but exhibits a minor effect on viremiain vivo. However, ribavirin significantly improves the disease outcome when administered in combination with sub-optimal doses of favipiravir, a strong antiviral drug. The mechanisms explaining these conflicting findings have not been determined, so far. Here, we used an interdisciplinary approach combining mathematical models and experimental data in LASV-infected mice that were treated with ribavirin alone or in combination with the drug favipiravir to explore different putative mechanisms of action for ribavirin. We test four different hypotheses that have been previously suggested for ribavirin’s mode of action: (i) acting as a mutagen, thereby limiting the infectivity of new virions; (ii) reducing viremia by impairing viral production; (iii) modulating cell damage, i.e., by reducing inflammation, and (iv) enhancing antiviral immunity. Our analysis indicates that enhancement of antiviral immunity, as well as effects on viral production or transmission are unlikely to be ribavirin’s main mechanism mediating its antiviral effectiveness against LASV infection. Instead, the modeled viral kinetics suggest that the main mode of action of ribavirin is to protect infected cells from dying, possibly reducing the inflammatory response. Lassa fever (LF) is a severe and often fatal hemorrhagic disease caused by Lassa virus (LASV), a member of the Arenaviridae virus family. -

Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Details Generic Name Indinavir Trade Name Crixivan® Class Protease Inhibitor Molecular Weight 711.88 (as sulphate) Structure HO OH N N H H SO N N 2 4 O HN O H3C CH3 H3C Summary of Key Pharmacokinetic Parameters Plasma half life 1.8 ± 0.4 h Cmax 8.97 ± 2.87 µg/ml (800 mg every 8 hours) Cmin 0.146 µg/ml (800 mg every 8 hours) AUC 21.82 ± 8.11 µg/ml.h (800 mg every 8 hours) Bioavailability Approx 65% Absorption Administration of indinavir with a meal high in calories, fat, and protein resulted in a blunted and reduced absorption with an approximate 80 % reduction in AUC and an 86 % reduction in Cmax. Administration with light meals (e.g., dry toast with jam or fruit conserve, apple juice, and coffee with skimmed or fat–free milk and sugar or corn flakes, skimmed or fat–free milk and sugar) resulted in plasma concentrations comparable to the corresponding fasted values. Protein Binding ~60% Volume of Distribution ~1.74 L/kg [1] CSF:Plasma ratio 0.14 [2] Semen:Plasma ratio 1.9 [2] Renal Clearance <20% as unchanged drug Renal Impairment Safety in patients with impaired renal function has not been studied; less than 20% of indinavir is excreted in the urine as unchanged drug or metabolites. Hepatic Impairment Safety and efficacy of indinavir has not been established in patients with significant underlying liver disorders and should be used with caution. -

Drug-Drug Interaction Between Protease Inhibitors and Statins and Proton Pump Inhibitors
Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Item Type text; Electronic Report Authors Orido, Charles; McKinnon, Samantha Publisher The University of Arizona. Rights Copyright © is held by the author. Download date 01/10/2021 01:48:07 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/636245 Group 47 :Orido/Samantha 1 Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Course Title: PhPr 862 Date: April 3, 2019 Faculty Advisor: Dr. Dan Malone Students: Charles Orido, Samantha McKinnon Pharm.D. Candidates, Class of 2019 Group 47 :Orido/Samantha 2 Objective The purpose of this article is to provide a systematic review of the pharmacokinetic and clinical data on drug-drug interactions between protease inhibitors (PIs) and statins, atazanavir and proton pump inhibitors (PPIs)and their clinical relevance. Methods A literature search was performed using Medline, EMBASE and google scholar, abstracts from 1970 to 2019 of major conferences were searched and FDA drug information package inserts of the manufacturer of every currently available PI was looked at. All data was summarized and verified by at least two investigators. Results A total of 246 references were identified, 8 of which were studies of pharmacokinetic and pharmacodynamics interactions between simvastatin, lovastatin and protease inhibitors and an additional 7 articles that provided pharmacokinetic of proton pump inhibitors and Atazanavir. Conclusions Protease inhibitors increases the AUC and Cmax of simvastatin by approximately 500% and 517% respectively. Therefore, simvastatin and Lovastatin are not recommended for a co-administration with a protease inhibitor. -

Lethal Mutagenesis of Hepatitis C Virus Induced by Favipiravir
RESEARCH ARTICLE Lethal Mutagenesis of Hepatitis C Virus Induced by Favipiravir Ana I. de AÂ vila1, Isabel Gallego1,2, Maria Eugenia Soria3, Josep Gregori2,3,4, Josep Quer2,3,5, Juan Ignacio Esteban2,3,5, Charles M. Rice6, Esteban Domingo1,2*, Celia Perales1,2,3* 1 Centro de BiologõÂa Molecular ªSevero Ochoaº (CSIC-UAM), Consejo Superior de Investigaciones CientõÂficas (CSIC), Campus de Cantoblanco, 28049, Madrid, Spain, 2 Centro de InvestigacioÂn BiomeÂdica en Red de Enfermedades HepaÂticas y Digestivas (CIBERehd), Barcelona, Spain, 3 Liver Unit, Internal Medicine, Laboratory of Malalties Hepàtiques, Vall d'Hebron Institut de Recerca-Hospital Universitari Vall d a11111 ÂHebron, (VHIR-HUVH), Universitat Autònoma de Barcelona, 08035, Barcelona, Spain, 4 Roche Diagnostics, S.L., Sant Cugat del ValleÂs, Spain, 5 Universitat AutoÂnoma de Barcelona, Barcelona, Spain, 6 Center for the Study of Hepatitis C, Laboratory of Virology and Infectious Disease, The Rockefeller University, New York, United States of America * [email protected] (ED); [email protected] (CP) OPEN ACCESS Abstract Citation: de AÂvila AI, Gallego I, Soria ME, Gregori J, Quer J, Esteban JI, et al. (2016) Lethal Lethal mutagenesis is an antiviral approach that consists in extinguishing a virus by an Mutagenesis of Hepatitis C Virus Induced by excess of mutations acquired during replication in the presence of a mutagen. Here we Favipiravir. PLoS ONE 11(10): e0164691. show that favipiravir (T-705) is a potent mutagenic agent for hepatitis C virus (HCV) during doi:10.1371/journal.pone.0164691 its replication in human hepatoma cells. T-705 leads to an excess of G ! A and C ! U tran- Editor: Ming-Lung Yu, Kaohsiung Medical sitions in the mutant spectrum of preextinction HCV populations. -

An Examination of COVID-19 Medications' Effectiveness
healthcare Review An Examination of COVID-19 Medications’ Effectiveness in Managing and Treating COVID-19 Patients: A Comparative Review Mahmoud Al-Masaeed 1,* , Mohammad Alghawanmeh 2, Ashraf Al-Singlawi 3 , Rawan Alsababha 4 and Muhammad Alqudah 1 1 Faculty of Health and Medicine, University of Newcastle, Callaghan 2308, Australia; [email protected] 2 Faculty of Pharmacy, Philadelphia University, Amman 19392, Jordan; [email protected] 3 Independent Scholar, Amman 11731, Jordan; [email protected] 4 School of nursing and Midwifery, Western Sydney University, Sydney 2560, Australia; [email protected] * Correspondence: [email protected] Abstract: Background: The review seeks to shed light on the administered and recommended COVID- 19 treatment medications through an evaluation of their efficacy. Methods: Data were collected from key databases, including Scopus, Medline, Google Scholar, and CINAHL. Other platforms included WHO and FDA publications. The review’s literature search was guided by the WHO Citation: Al-Masaeed, M.; solidarity clinical trials for COVID-19 scope and trial-assessment parameters. Results: The findings Alghawanmeh, M.; Al-Singlawi, A.; indicate that the use of antiretroviral drugs as an early treatment for COVID-19 patients has been Alsababha, R.; Alqudah, M. An useful. It has reduced hospital time, hastened the clinical cure period, delayed and reduced the Examination of COVID-19 need for mechanical and invasive ventilation, and reduced mortality rates. The use of vitamins, Medications’ Effectiveness in minerals, and supplements has been linked to increased immunity and thus offering the body a Managing and Treating COVID-19 fighting chance. Nevertheless, antibiotics do not correlate with improving patients’ wellbeing and Patients: A Comparative Review.