ISENTRESS (Raltegravir) for Oral Suspension ------CONTRAINDICATIONS ------Initial U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Enzyme Inducers Efavirenz and Tipranavir/Ritonavir on the Pharmacokinetics of the HIV Integrase Inhibitor Dolutegravir
Eur J Clin Pharmacol (2014) 70:1173–1179 DOI 10.1007/s00228-014-1732-8 CLINICAL TRIAL Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir Ivy Song & Julie Borland & Shuguang Chen & Phyllis Guta & Yu Lou & David Wilfret & Toshihiro Wajima & Paul Savina & Amanda Peppercorn & Stephen Castellino & David Wagner & Louise Hosking & Michael Mosteller & Justin P.Rubio & Stephen C. Piscitelli Received: 7 May 2014 /Accepted: 11 August 2014 /Published online: 23 August 2014 # The Author(s) 2014. This article is published with open access at Springerlink.com Abstract study due to increases in alanine aminotransferase that were Purpose Dolutegravir (DTG) is an unboosted, integrase in- considered related to TPV/r. Co-administration with EFV hibitor for the treatment of HIV infection. Two studies evalu- resulted in decreases of 57, 39 and 75 % in DTG AUC(0–τ), ated the effects of efavirenz (EFV) and tipranavir/ritonavir Cmax and Cτ, respectively. Co-administration with TPV/r re- (TPV/r) on DTG pharmacokinetics (PK) in healthy subjects. sulted in decreases of 59, 46 and 76 % in DTG AUC(0–τ), Cmax Methods The first study was an open-label crossover where and Cτ, respectively. 12 subjects received DTG 50 mg every 24 hours (q24h) for Conclusions Given the reductions in exposure and PK/ 5 days, followed by DTG 50 mg and EFV 600 mg q24h for pharmacodynamic relationships in phase II/III trials, DTG 14 days. The second study was an open-label crossover where should be given at an increased dose of 50 mg twice daily 18 subjects received DTG 50 mg q24h for 5 days followed by when co-administered with EFV or TPV/r, and alternative TPV/r 500/200 mg every 12 hours (q12h) for 7 days and then regimens without inducers should be considered in integrase DTG 50 mg q24h and TPV/r 500/200 mg q12h for a further inhibitor-resistant patients. -

Revised 4/1/2021 GEORGIA MEDICAID FEE-FOR-SERVICE HIV
GEORGIA MEDICAID FEE-FOR-SERVICE HIV-AIDS PA SUMMARY Preferred (may not be all inclusive) Non-Preferred Abacavir generic Abacavir/lamivudine/zidovudine generic Abacavir/lamivudine generic Aptivus (tipranavir) Complera (emtricitabine/rilpivirine/tenofovir disoproxil Atazanavir capsules generic fumarate) Atripla (efavirenz/emtricitabine/tenofovir disoproxil Crixivan (indinavir) fumarate) Biktarvy (bictegravir/emtricitabine/tenofovir Delstrigo (doravirine/lamivudine/tenofovir disoproxil alafenamide) fumarate) Cimduo (lamivudine/tenofovir disoproxil fumarate) Fuzeon (enfuvirtide) Descovy (emtricitabine/tenofovir alafenamide) Intelence (etravirine) Dovato Invirase (saquinavir) Edurant (rilpivirine)* Lexiva (fosamprenavir) Efavirenz tablets generic Nevirapine extended-release generic Emtriva (emtricitabine) Norvir Powder (ritonavir) Epivir solution (lamivudine) Pifeltro (doravirine) Evotaz (atazanavir/cobicistat)* Reyataz Powder (atazanavir) Genvoya (elvitegravir/cobicistat/emtricitabine/ Ritonavir tablets generic tenofovir alafenamide) Isentress and Isentress HD (raltegravir)* Rukobia (fostemsavir) Juluca (dolutegravir/rilpivirine) Selzentry (maraviroc) Kaletra (lopinavir/ritonavir) Stavudine generic^ Stribild (elvitegravir/cobicistat/emtricitabine/ tenofovir Lamivudine generic disoproxil fumarate) Symfi (efavirenz 600 mg/lamivudine/tenofovir Lamivudine/zidovudine generic disoproxil fumarate) Symfi Lo (efavirenz 400 mg/lamivudine/tenofovir Nevirapine immediate-release tablets generic disoproxil fumarate) Norvir (ritonavir) Temixys (lamivudine/tenofovir -

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

Recommendations for the Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection Table of Contents Table 1
Recommendations for the Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection Table of Contents Table 1. Outline of the Guidelines Development Process..........................................................................................................................1 Table 2. Rating Scheme for Recommendations........................................................................................................................................3 Table 3. Sample Schedule for Clinical and Laboratory Monitoring of Children Before and After Initiation of Combination Antiretroviral Therapy .................................................................................................................4 Table 4. Primary FDA-Approved Assays for Monitoring Viral Load D-8 Table 5. HIV Infection Stage Based on Age-Specific CD4 Count or Percentage ........................................................................................4 Table 6. HIV-Related Symptoms and Conditions ......................................................................................................................................5 Table 7. Antiretroviral Regimens Recommended for Initial Therapy for HIV Infection in Children ...........................................................................................................................................................................................7 Table 8. Advantages and Disadvantages of Antiretroviral Components Recommended for Initial Therapy in Children ............................................................................................................................................................10 -

Vitekta Pi.Pdf
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------DOSAGE FORMS AND STRENGTHS-------------------- These highlights do not include all the information needed to use Tablets: 85 mg and 150 mg (3) VITEKTA safely and effectively. See full prescribing information for VITEKTA. --------------------------------CONTRAINDICATIONS------------------------------ VITEKTA® (elvitegravir) tablets, for oral use There are no contraindications to VITEKTA. (4) Initial U.S. Approval: 2012 Due to the need to use VITEKTA with a protease inhibitor coadministered with ritonavir, consult prescribing information of -------------------------------INDICATIONS AND USAGE------------------------- coadministered protease inhibitor and ritonavir for their VITEKTA is a human immunodeficiency virus type 1 (HIV-1) integrase contraindications. (4) strand transfer inhibitor used in combination with an HIV protease inhibitor coadministered with ritonavir and with other antiretroviral -------------------------WARNINGS AND PRECAUTIONS---------------------- drug(s) indicated for the treatment of HIV-1 infection in antiretroviral Do not use with protease inhibitors coadministered with cobicistat. treatment-experienced adults. (1) (5.2) Do not use with other elvitegravir-containing drugs, including Limitations of Use: STRIBILD. (5.2) There are no comparative pharmacokinetic or clinical data Immune reconstitution syndrome: May necessitate further evaluation evaluating VITEKTA with cobicistat as single entities compared to and treatment. (5.3) STRIBILD®. (1) --------------------------------ADVERSE REACTIONS----------------------------- VITEKTA coadministered with protease inhibitors and cobicistat is The most common adverse drug reaction to VITEKTA (all grades) is not recommended. (1) diarrhea. (6.1) Coadministration of VITEKTA with dosage regimens or HIV-1 protease inhibitors other than those presented in Table 1 is not To report SUSPECTED ADVERSE REACTIONS, contact Gilead recommended. (1) Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
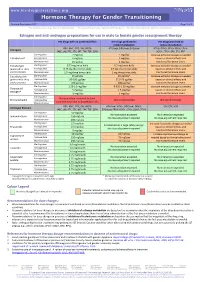
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -

United States Patent (10) Patent No.: US 7,244,716 B2 Klaes Et Al
USOO724471.6B2 (12) United States Patent (10) Patent No.: US 7,244,716 B2 Klaes et al. (45) Date of Patent: Jul. 17, 2007 (54) PHARMACEUTICAL COMPOSITION OF 2001/0036920 A1* 11/2001 Hirschman ................... 514f14 ANTIVIRAL AGENTS FOREIGN PATENT DOCUMENTS (75) Inventors: Heinz-Gerd Klaes, Gau-Bickelheim WO WO 88.00050 1, 1988 (DE); Elena Koundourakis, Danbury, WO WO 91/O1137 2, 1991 CT (US); Hernan Valdez, Somers, NY WO WO 99,09031 2, 1999 (US); Douglas Lytle Mayers, WO WO 99,41268 8, 1999 Newtown, CT (US) WO WOOO, 51641 9, 2000 (73) Assignee: Boehringer Ingelheim International OTHER PUBLICATIONS GmbH, Ingelheim (DE) Roy M. Gulicket al; New drugs for HIV therapy: AIDS (2002) vol. (*)c Notice:- r Stil tO E. state th SMR t Erik16 No. De 4 Clercq;pp. S135-S144; New Developments Lippincott Williamsin Anti-HIV & Wilkins. Chemotherapy; patent 1s extended or adjusted under Current Medicinal Chemistry (2001) vol. 8 pp. 1543-1572; U.S.C. 154(b) by 0 days. Bentham Science Publishers Ltd. (21) Appl. No.: 10/809,060 * cited by examiner (22) Filed: Mar 25, 2004 Primary Examiner Patrick Lewis 9 (74) Attorney, Agent, or Firm Michael P. Morris; (65) Prior Publication Data Mary-Ellen M. Devlin US 2004/O235779 A1 Nov. 25, 2004 (57) ABSTRACT (30) Foreign Application Priority Data In accordance with the present invention there is provided a Mar. 27, 2003 (EP) .................................. O3OO7OO1 pharmaceutical composition useful for the treatment or Jul. 17, 2003 (EP) ... ... O3O16224 prophylaxis of viral infections comprising tipranavir and at Dec. 20, 2003 (EP) .................................. O3O29507 least one antiviral active compound of formula (I) (51) Int. -

Review CCR5 Antagonists: Host-Targeted Antiviral Agents for the Treatment of HIV Infection, 4 Years On
Antiviral Chemistry & Chemotherapy 2010 20:179–192 (doi: 10.3851/IMP1507) Review CCR5 antagonists: host-targeted antiviral agents for the treatment of HIV infection, 4 years on Mike Westby1* and Elna van der Ryst1 1Pfizer Global Research and Development, Sandwich, Kent, UK *Corresponding author: e-mail: [email protected] The chemokine coreceptor 5 (CCR5) antagonists are anti- are in various stages of clinical development. In 2005, we retroviral agents with an extracellular, host-targeted reviewed the limited clinical data then available on CCR5 mechanism of action against HIV. Maraviroc, the first- antagonists. In this follow-up review, we revisit the field and in-class CCR5 antagonist, received regulatory approval in assess the clinical and virological data that have emerged 2007, becoming the first oral antiretroviral from a new in the 4 years since, with particular reference to maraviroc class in more than 10 years. Other compounds in this class for which the most comprehensive data currently exist. Introduction Chemokine coreceptor 5 (CCR5) antagonists are a new supporting the current and future role of small-molecule class of antiretroviral agents that inhibit HIV replica- CCR5 antagonists in the treatment of HIV infection. tion by binding to CCR5 on the surface of host target cells and preventing entry of viral strains reliant on this Status of CCR5 antagonists in clinical coreceptor for infection. The first and only currently development in 2005 approved agent within this class, maraviroc, received marketing authorisation in the USA, Europe and other Of the three small-molecule CCR5 antagonists in regions of the world in 2007, with an indication for clinical development in 2005, only maraviroc (Figure treatment-experienced adult patients with only CCR5- 1A) is currently approved for clinical use.