Seed Survival of Australian Acacia in the Western Cape of South Africa in the Presence of Biological Control Agents and Given Environmental Variation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plants Prohibited from Sale in South Australia Plants Considered As Serious Weeds Are Banned from Being Sold in South Australia
Plants prohibited from sale in South Australia Plants considered as serious weeds are banned from being sold in South Australia. Do not buy or sell any plant listed as prohibited from sale. Plants listed in this document are declared serious weeds and are prohibited from sale anywhere in South Australia pursuant to Section 188 of the Landscape South Australia Act 2019 (refer South Australian Government Gazette 60: 4024-4038, 23 July 2020). This includes the sale of nursery stock, seeds or other propagating material. However, it is not prohibited to sell or transport non-living products made from these plants, such as timber from Aleppo pine, or herbal medicines containing horehound. Such products are excluded from the definition of plant, under the Landscape South Australia (General) Regulations 2020. Section 3 of the Act defines sell as including: • barter, offer or attempt to sell • receive for sale • have in possession for sale • cause or permit to be sold or offered for sale • send, forward or deliver for sale • dispose of by any method for valuable consideration • dispose of to an agent for sale on consignment • sell for the purposes of resale How to use this list Plants are often known by many names. This list can help you to find the scientific name and other common names of a declared plant. This list is not intended to be a complete synonymy for each species, such as would be found in a taxonomic revision. The plants are listed alphabetically by the common name as used in the declaration. Each plant is listed in the following format: Common name alternative common name(s) Scientific name Author Synonym(s) Author What to do if you suspect a plant for sale is banned If you are unsure whether a plant offered for sale under a particular name is banned, please contact your regional landscape board or PIRSA Biosecurity SA. -

(Hymenoptera: Eurytomidae) in the Integrated Control of Acacia Species in South Africa
Proceedings of the X International Symposium on Biological Control of Weeds 919 4-14 July 1999, Montana State University, Bozeman, Montana, USA Neal R. Spencer [ed.]. pp. 919-929 (2000) The Potential Role of Bruchophagus acaciae (Cameron) (Hymenoptera: Eurytomidae) in the Integrated Control of Acacia Species in South Africa R. L. HILL1, A. J. GORDON2, and S. NESER3 1Richard Hill & Associates, Private Bag 4704, Christchurch, New Zealand 2Plant Protection Research Institute, Private Bag X5017, Stellenbosch, 7599 South Africa 3Plant Protection Research Institute, Private Bag X134, Pretoria, 0001 South Africa Abstract Australian acacias invade watersheds and riverbeds in South Africa, reducing water flows and threatening environmental and economic values. Acacia mearnsii is the most widespread and important weed but also forms the basis of an important industry. A. dealbata, and to a lesser extent A. decurrens are also problems. All belong to the Section Botrycephalae of the sub-genus Heterophyllum. Short term control is achieved locally by removing plants, and by using herbicides, but seed-feeding control agents may provide an acceptable solution in the long term. Larvae of Bruchophagus acaciae (Cameron) (Hymenoptera: Eurytomidae) develop in the seeds of acacias. It was described from New Zealand, but is an Australian species. We explore whether B. acaciae has a role as a con- trol agent for acacias in South Africa. Seed was collected from 28 Australian species of Acacia growing in New Zealand. Attack was restricted to four of the seven species with- in the Section Botrycephalae, and two cases of attack on Acacia rubida (Section Phyllodineae; n=9). Apart from a wasp reared from one seed, A. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

Pine As Fast Food: Foraging Ecology of an Endangered Cockatoo in a Forestry Landscape
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Online @ ECU Edith Cowan University Research Online ECU Publications 2013 2013 Pine as Fast Food: Foraging Ecology of an Endangered Cockatoo in a Forestry Landscape William Stock Edith Cowan University, [email protected] Hugh Finn Jackson Parker Ken Dods Follow this and additional works at: https://ro.ecu.edu.au/ecuworks2013 Part of the Forest Biology Commons, and the Terrestrial and Aquatic Ecology Commons 10.1371/journal.pone.0061145 Stock, W.D., Finn, H. , Parker, J., & Dods, K. (2013). Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape. PLoS ONE, 8(4), e61145. Availablehere This Journal Article is posted at Research Online. https://ro.ecu.edu.au/ecuworks2013/1 Pine as Fast Food: Foraging Ecology of an Endangered Cockatoo in a Forestry Landscape William D. Stock1*, Hugh Finn2, Jackson Parker3, Ken Dods4 1 Centre for Ecosystem Management, Edith Cowan University, Joondalup, Western Australia, Australia, 2 School of Biological Sciences and Biotechnology, Murdoch University, Perth, Western Australia, Australia, 3 Department of Agriculture and Food, Western Australia, South Perth, Western Australia, Australia, 4 ChemCentre, Bentley, Western Australia, Australia Abstract Pine plantations near Perth, Western Australia have provided an important food source for endangered Carnaby’s Cockatoos (Calyptorhynchus latirostris) since the 1940s. Plans to harvest these plantations without re-planting will remove this food source by 2031 or earlier. To assess the impact of pine removal, we studied the ecological association between Carnaby’s Cockatoos and pine using behavioural, nutritional, and phenological data. -

Acacia Saligna RA
Risk Assessment: ………….. ACACIA SALIGNA Prepared by: Etienne Branquart (1), Vanessa Lozano (2) and Giuseppe Brundu (2) (1) [[email protected]] (2) Department of Agriculture, University of Sassari, Italy [[email protected]] Date: first draft 01 st November 2017 Subsequently Reviewed by 2 independent external Peer Reviewers: Dr Rob Tanner, chosen for his expertise in Risk Assessments, and Dr Jean-Marc Dufor-Dror chosen for his expertise on Acacia saligna . Date: first revised version 04 th January 2018, revised in light of comments from independent expert Peer Reviewers. Approved by the IAS Scientific Forum on 26/10/2018 1 2 3 4 5 6 7 1 Branquart, Lozano & Brundu PRA Acacia saligna 8 9 10 Contents 11 Summary of the Express Pest Risk Assessment for Acacia saligna 4 12 Stage 1. Initiation 6 13 1.1 - Reason for performing the Pest Risk Assessment (PRA) 6 14 1.2 - PRA area 6 15 1.3 - PRA scheme 6 16 Stage 2. Pest risk assessment 7 17 2.1 - Taxonomy and identification 7 18 2.1.1 - Taxonomy 7 19 2.1.2 - Main synonyms 8 20 2.1.3 - Common names 8 21 2.1.4 - Main related or look-alike species 8 22 2.1.5 - Terminology used in the present PRA for taxa names 9 23 2.1.6 - Identification (brief description) 9 24 2.2 - Pest overview 9 25 2.2.2 - Habitat and environmental requirements 10 26 2.2.3 Resource acquisition mechanisms 12 27 2.2.4 - Symptoms 12 28 2.2.5 - Existing PRAs 12 29 Socio-economic benefits 13 30 2.3 - Is the pest a vector? 14 31 2.4 - Is a vector needed for pest entry or spread? 15 32 2.5 - Regulatory status of the pest 15 33 2.6 - Distribution -

Human-Mediated Introductions of Australian Acacias
Diversity and Distributions, (Diversity Distrib.) (2011) 17, 771–787 S EDITORIAL Human-mediated introductions of PECIAL ISSUE Australian acacias – a global experiment in biogeography 1 2 1 3,4 David M. Richardson *, Jane Carruthers , Cang Hui , Fiona A. C. Impson , :H Joseph T. Miller5, Mark P. Robertson1,6, Mathieu Rouget7, Johannes J. Le Roux1 and John R. U. Wilson1,8 UMAN 1 Centre for Invasion Biology, Department of ABSTRACT - Botany and Zoology, Stellenbosch University, MEDIATED INTRODUCTIONS OF Aim Australian acacias (1012 recognized species native to Australia, which were Matieland 7602, South Africa, 2Department of History, University of South Africa, PO Box previously grouped in Acacia subgenus Phyllodineae) have been moved extensively 392, Unisa 0003, South Africa, 3Department around the world by humans over the past 250 years. This has created the of Zoology, University of Cape Town, opportunity to explore how evolutionary, ecological, historical and sociological Rondebosch 7701, South Africa, 4Plant factors interact to affect the distribution, usage, invasiveness and perceptions of a Protection Research Institute, Private Bag globally important group of plants. This editorial provides the background for the X5017, Stellenbosch 7599, South Africa, 20 papers in this special issue of Diversity and Distributions that focusses on the 5Centre for Australian National Biodiversity global cross-disciplinary experiment of introduced Australian acacias. A Journal of Conservation Biogeography Research, CSIRO Plant Industry, GPO Box Location Australia and global. 1600, Canberra, ACT, Australia, 6Department of Zoology and Entomology, University of Methods The papers of the special issue are discussed in the context of a unified Pretoria, Pretoria 0002, South Africa, framework for biological invasions. -

Seeding Victoria Inc
Seeding Victoria Inc Ballarat Region Seed Bank Back to catalogue search Sawpit Rd, Creswick. General Stock P.O. Box 3., Creswick, 3363 Jul-02 Phone: 03 5345 2200 Fax: 03 53451357 e-mail:[email protected] Servicing: North Central, Corangamite, Wimmera and parts of Glenelg-Hopkins and Port Phillip catchments Note when ordering - there is a $5 batch handling charge for each batch ordered in addition to the price per kg of seed. Prices quoted in this catalogue do not include GST. Packaging and postage charge will apply to posted orders. Seed prices within the same species may vary due to variation in the seed germination, age, cleanliness and quality control considerations. Batch Botanical Name Common Name Provenance Gms Price does not include GST $ 1kg 11450 Acacia acinacea Gold-dust Wattle Barfold 970.00 $660.00 11054 Acacia acinacea Gold-dust Wattle Bealiba 862.00 $660.00 11285 Acacia acinacea Gold-dust Wattle Bealiba 445.00 $660.00 11266 Acacia acinacea Gold-dust Wattle Bourke's Flat 345.00 $660.00 10906 Acacia acinacea Gold-dust Wattle Carapooee 2950.00 $660.00 11227 Acacia acinacea Gold-dust Wattle Castlemaine 540.00 $660.00 11278 Acacia acinacea Gold-dust Wattle Dunolly 398.00 $660.00 11298 Acacia acinacea Gold-dust Wattle Dunolly 1400.00 $660.00 11453 Acacia acinacea Gold-dust Wattle Glenhope/White Hills 480.00 $660.00 10115 Acacia acinacea Gold-dust Wattle Gowar 1975.00 $660.00 10204 Acacia acinacea Gold-dust Wattle Gowar 7425.00 $660.00 4695 Acacia acinacea Gold-dust Wattle Kamarooka 55.00 $400.00 11451 Acacia acinacea Gold-dust -

A Review of Pharmacological Activities of Acacia Nilotica (Linn) Willd W.S.R to Osteoporosis Kamini Kaushal 1 Abstract
Review Article A Review of Pharmacological Activities of Acacia nilotica (Linn) willd W.S.R to Osteoporosis Kamini Kaushal 1 Abstract Medicinal plants have been utilized for the treatment of diseases since creation of earth or before it; in traditional medicine, they still play an important role as effective and have natural origin. Acacia nilotica commonly known as babul belongs to the family Fabaceae and is widely distributed all over India, SriLanka, and Sudan; Egypt is the native country of this plant. Useful parts such as root, bark, leaves, flower, gum, pods, etc., are used in medicines. Different parts of the plant like leaves and fruit contain tannin; flower contains stearic acid, kaempferol-3-glucoside, isoquercetin, leucocyanidin; pod contains tannin, polyphenolic compounds, gum Contains arabic acid combined with calcium, magnesium and potassium. In traditional medicine, it is used for bleeding diseases, prolapsed, leucorrhoea, antihypertensive, antispasmodic, antibacterial, antifungal, antioxidant activity, etc. The present review is an attempt to explore and comprehensively highlight use for osteoporosis, phytochemical properties and pharmacological uses of A. nilotica reported till date. Keywords: Acacia arabica; Acacia nilotica , Pharmacological study, Tannin, Arabic acid, Uses Introduction More than 30% healthcare industry from all over the world relies on medicinal plants. In India, traditional system of medicine such as Ayurveda since ancient times is root of medicine. About 750 species are being utilized in Ayurveda system of medicine and in modern medicine around 30 species only.1 Medicinal plants are playing continuously essential part of Indian system of medicine. Acacia genus belongs to shrubs and trees, subfamily Mimosoideae, 2,3 of the family Fabaceae (Leguminosae).4,5 The species name is nilotica/arebica/scorpioides. -

Restricted Invasive Plants of Queensland
Restricted invasive plants Restricted invasive plants of Queensland Restricted invasive plants of Queensland Hudson pear (Cylindropuntia rosea syn. Cylindropuntia pallida) Fireweed (Senecio madagascariensis) Mother-of-millions (Kalanchoe delagoense) Bunny ears (Opuntia microdasys) The new Biosecurity Act The Biosecurity Act 2014 protects Queensland’s economy, Species not listed as restricted may be listed as prohibited biodiversity and people’s lifestyles from the threats posed under the Act or may be listed by a local government level by invasive pests and diseases under local laws. Under the Act, certain species of invasive plants are listed Australian Government legislation administered by the as ‘restricted’ biosecurity matter. Australian Department of Agriculture also applies to the import of all plants into Australia. What is restricted matter? • Mexican bean tree (Cecropia pachystachya, C. palmata and C. peltata) Restricted matter is listed in the Act and includes a range • Mexican feather grass (Nassella tenuissima) of invasive plants that are present in Queensland. These invasive plants are having significant adverse impacts • miconia (M. calvescens, M. cionotricha, M. nervosa in Queensland and it is desirable to manage them and and M. racemosa) prevent their spread, thereby protecting un-infested • mikania vine (Mikania micrantha) parts of the State. • mimosa pigra (Mimosa pigra) The Act requires everyone to take all reasonable and practical measures to minimise the biosecurity risks • bunny ears (Opuntia microdasys) associated with invasive plants and animals under • riverina prickly pear (Opunita elata) their control. This is called a general biosecurity obligation (GBO). • water mimosa (Neptunia oleracea and N. plena). The specific restriction requirements also apply to a Restricted invasive plants that are person when dealing with restricted invasive matter. -

Early Growth and Survival of Different Woody Plant Species Established Through Direct Sowing in a Degraded Land, Southern Ethiopia
JOURNAL OF DEGRADED AND MINING LANDS MANAGEMENT ISSN: 2339-076X (p); 2502-2458 (e), Volume 6, Number 4 (July 2019):1861-1873 DOI:10.15243/jdmlm.2019.064.1861 Research Article Early growth and survival of different woody plant species established through direct sowing in a degraded land, Southern Ethiopia Shiferaw Alem*, Hana Habrova Department of Forest Botany, Dendrology and Geo-biocenology, Mendel University in Brno, Zemedelska 3/61300, Brno, Czech Republic *corresponding author: [email protected] Received 2 May 2019, Accepted 30 May 2019 Abstract: In addition to tree planting activities, finding an alternative method to restore degraded land in semi-arid areas is necessary, and direct seeding of woody plants might be an alternative option. The objectives of this study paper were (1) evaluate the growth, biomass and survival of different woody plant species established through direct seeding in a semi-arid degraded land; (2) identify woody plant species that could be further used for restoration of degraded lands. To achieve the objectives eight woody plant species seeds were gathered, their seeds were sown in a degraded land, in a randomized complete block design (RCBD) (n=4). Data on germination, growth and survival of the different woody plants were collected at regular intervals during an eleven-month period. At the end of the study period, the remaining woody plants' dry biomasses were assessed. One-way analysis of variance (ANOVA) was used for the data analysis and mean separation was performed using Fisher’s least significant difference (LSD) test (p=0.05). The result revealed significant differences on the mean heights, root length, root collar diameters, root to shoot ratio, dry root biomasses and dry shoot biomasses of the different species (p < 0.05). -

Acacias and Galls. Acaciaelongifoliae
Australian Native Plants Society (Australia) Inc. ACACIA STUDY GROUP NEWSLETTER Group Leader and Newsletter Editor Seed Bank Curator Bill Aitchison Victoria Tanner 13 Conos Court, Donvale, Vic 3111 Phone (03) 98723583 Email: [email protected] Acacia brunioides No. 139 December 2017 ISSN 1035-4638 From The Leader Contents Page Dear Members From the Leader 1 As I write this short message in December, typically for this Welcome 2 time of year there are few wattles in flower in our garden – From Members and Readers 2 Acacia implexa and A. muelleriana are the two exceptions, Acacia cretacea 5 together with just a few flowers on A. deanei. But at this Acacia pruinosa 6 time of year, it is never the wattle flowers that create the Acacia rhetinocarpa 6 interest, but rather the seeds that have matured. Acacias and Galls 8 Archibald James Campbell 9 There are always some interesting observations in relation Xylella fastidiosa 9 to seeds. For example, I find it interesting that seeds from Wattle Family Plumbing 10 last year’s flowering of both A. implexa and A. muelleriana Use of Acacia dealbata in dyeing 10 mature at the same time as this year’s flowering, so they Seed Bank 10 have taken close to a year to come to maturity. In relation Study Group Membership 11 to A. implexa, there has been a carpet of seeds lying on the Financial Report 2016-17 11 ground underneath the trees – but then when one looks into Seed Bank Listing 12 the canopy of the trees, noisy rainbow lorikeets are obviously enjoying the seeds, at least their white fleshy arils. -
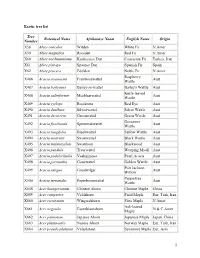
1 Exotic Tree List Tree Number Botanical Name Afrikaanse Naam
Exotic tree list Tree Botanical Name Afrikaanse Naam English Name Origin Number X58 Abies concolor Witden White Fir N.Amer X59 Abies magnifica Rooiden Red Fir N.Amer X60 Abies nordmanniana Kaukasiese Den Caucasian Fir Turkey, Iran X61 Abies pinsapo Spaanse Den Spanish Fir Spain X62 Abies procera Edelden Noble Fir N.Amer Raspberry X486 Acacia acuminata Frambosewattel Aust Wattle X487 Acacia baileyana Bailey-se-wattel Bailey's Wattle Aust Knife-leaved X488 Acacia cultriformis Mesblaarwattel Aust Wattle X489 Acacia cyclops Rooikrans Red Eye Aust X490 Acacia dealbata Silwerwattel Silver Wattle Aust X491 Acacia decurrens Groenwattel Green Wattle Aust Gossamer X492 Acacia floribunda Spinnerakwattel Aust Wattle X493 Acacia longifolia Bleekwattel Sallow Wattle Aust X494 Acacia mearnsii Swartwattel Black Wattle Aust X495 Acacia melanoxylon Swarthout Blackwood Aust X496 Acacia pendula Treurwattel Weeping Myall Aust X497 Acacia podalyriifolia Vaalmimosa Pearl Acacia Aust X498 Acacia pycnantha Gouewattel Golden Wattle Aust Port Jackson X499 Acacia saligna Goudwilger Aust Willow Peppertree X500 Acacia terminalis Peperboomwattel Aust Wattle X658 Acer buergerianum Chinese Ahorn Chinese Maple China X659 Acer campestre Veldahorn Field Maple Eur, Turk, Iran X660 Acer circinatum Wingerdahorn Vine Maple N Amer Ash-leaved X661 Acer negundo Essenblaarahorn N & C Amer Maple X662 Acer palmatum Japanse Ahorn Japanese Maple Japan, China X663 Acer platanoides Noorse Ahorn Norway Maple Eur, Turk, Iran X664 Acer pseudo-platanus Valsplataan Sycamore Maple Eur, Asia