Albizia Amara (Roxb.) Boiv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -
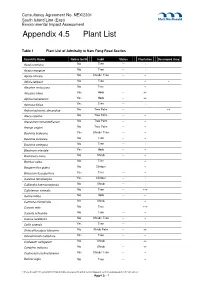
Appendix 4.5 Plant List
Consultancy Agreement No. NEX/2301 South Island Line (East) Environmental Impact Assessment Appendix 4.5 Plant List Table 1 Plant List of Admiralty to Nam Fung Road Section Scientific Name Native to HK Habit Status Plantation Developed Area Acacia confusa No Tree -- + Acacia mangium No Tree -- + Aglaia odorata No Shrub / Tree -- + Albizia lebbeck No Tree -- + + Aleurites moluccana No Tree -- + Alocasia odora Yes Herb -- ++ Alpinia hainanensis Yes Herb -- ++ Aporusa dioica Yes Tree -- + Archontophoenix alexandrae No Tree Palm -- ++ Areca catechu No Tree Palm -- + Arecastrum romanzoffianum No Tree Palm -- + Arenga engleri No Tree Palm -- + Bauhinia blakeana Yes Shrub / Tree -- + Bauhinia purpurea No Tree -- + Bauhinia variegata No Tree -- + Blechnum orientale Yes Herb -- + Boehmeria nivea No Shrub -- + Bombax ceiba No Tree -- + Bougainvillea glabra No Climber -- + Broussonetia papyrifera Yes Tree -- + Calamus tetradactylus Yes Climber -- + Calliandra haematocephala No Shrub -- + Callistemon viminalis No Tree -- +++ Canna indica No Herb -- + Carmona microphylla No Shrub -- + Caryota mitis No Tree -- +++ Caryota ochlandra No Tree -- + Cassia surattensis No Shrub / Tree -- + Celtis sinensis Yes Tree -- + Chrysalidocarpus lutescens No Shrub Palm -- ++ Cinnamomum camphora Yes Tree -- + Codiaeum variegatum No Shrub -- ++ Cordyline fruticosa No Shrub -- ++ Cratoxylum cochinchinense Yes Shrub / Tree -- + Delonix regia No Tree -- + P:\Hong Kong\INF\Projects2\248137 SIL(E) EIA\Deliverables\Final EIA Vol I\3rd\Appendices\4 Ecology\Appendix 4.5 Plant -

Albizia Amara - a Potential Medicinal Plant: a Review
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2014): 5.611 Albizia amara - A Potential Medicinal Plant: A Review G. Indravathi1, 2, R. Sreekanth Reddy3, Pakala Suresh Babu3 1Department of Biotechnology, K.V.R. Govt. College for Women, Kurnool-518 002, Andhra Pradesh, India 2Department of Biotechnology, Jawaharlal Nehru Technological University, Anantapur-515 001, Andhra Pradesh, India 3Department of Biochemistry, Sri Krishnadevaraya University, Anantapur, Andhra Pradesh-515 001, India Abstract: Albizia amara is an important medicinal plant found throughout India. The entire plant possesses pharmaceutical constituents of great significance. The present article gives an update on bioactive compounds and medicinal importance of Albizia amara. This plant has been used as an important folk medicine for the treatment of several diseases like diarrhea, gonorrhea, skin diseases, poisonous bites and leprosy. Further, phytochemical investigation revealed the presence of wide variety of bioactive compounds such as macrocyclic spermine alkaloids, triterpene saponins, phenols, flavonyl glycosides, tannins, sterols in the plant extract of A. amara. In addition, the plant extract possess the pharmacological properties like anticancer, antihyperlipidimic, antiinflammatory, antimicrobial, analgesic and antioxidant activities. Because of the presence of several phytoconstituents, pharmacological activities and wide distribution, this will be an ideal plant resource for the treatment of several endemic diseases. Keywords: Albizia amara, Medicinal Plant, Bioactive compounds,Pharmacological Properties 1. Introduction is present in the dry regions of Tamil Nadu, Andhra Pradesh and Karnataka (Chakrabarthy T et al., 1996). The genuses Albizia is represented by more than 100 species and are mainly confined to tropical and sub- tropical regions Morphology of Asia, Africa and Australia. -

Southwest Guangdong, 28 April to 7 May 1998
Report of Rapid Biodiversity Assessments at Fusui Rare Animal Nature Reserve, Southwest Guangxi, China, 1998 and 2001 Kadoorie Farm and Botanic Garden in collaboration with Guangxi Forestry Department Guangxi Institute of Botany Guangxi Normal University April 2002 South China Forest Biodiversity Survey Report Series: No. 12 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Fusui Rare Animal Nature Reserve, Southwest Guangxi, China, 1998 and 2001 Editors John R. Fellowes, Michael W.N. Lau, Billy C.H. Hau, Ng Sai-Chit and Bosco P.L. Chan Contributors Kadoorie Farm and Botanic Garden: Bosco P.L. Chan (BC) John R. Fellowes (JRF) Billy C.H.Hau (BH) Michael W.N. Lau (ML) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Graham T. Reels (GTR) Guangxi Institute of Botany: Wei Fanan (WFN) Zou Xiangui (ZXG) Guangxi Normal University: Lu Liren (LLR) Voluntary consultants: Geoff J. Carey (GJC) Paul J. Leader (PJL) Keith D.P. Wilson (KW) Background The present report details the findings of a trip to Southwest Guangxi by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme. The overall aim of the programme is to minimise the loss of forest biodiversity in the region, and the emphasis in the first phase is on gathering up-to-date information on the distribution and status of fauna and flora. Citation Kadoorie Farm and Botanic Garden, 2002. Report of Rapid Biodiversity Assessments at Fusui Rare Animal Nature Reserve, Southwest Guangxi, China, 1998 and 2001. South China Forest Biodiversity Survey Report Series (Online Simplified Version): No. -

Agroforestry in Rice-Production Landscapes in Southeast Asia a Practical Manual
Agroforestry in rice-production landscapes in Southeast Asia a practical manual Agroforestry in rice-production landscapes in Southeast Asia a practical manual Editors Prasit Wangpakapattanawong Robert Finlayson Ingrid Öborn James M. Roshetko Fergus Sinclair Kenichi Shono Simone Borelli Anique Hillbrand Michela Conigliaro This manual is intended to help rural advisory and agricultural extension workers guide farming communities in the establishment of agroforestry practices in rice-production landscapes in Southeast Asia. It sets out the steps to be taken to successfully integrate trees in rice-fields and associated farms and landscapes and presents practical tools that can be used by extensionists when supporting farmers who are implementing agroforestry practices on their farms. The ultimate aim of this guide is to support farmers in increasing the overall productivity of their farms while increasing resilience to climate change, improving the health of the surrounding environment, and enhancing the livelihoods of their communities. Published by Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific World Agroforestry Centre (ICRAF) 2017 Wangpakapattanawong, P., Finlayson, R., Öborn, I., Roshetko, J.M., Sinclair, F., Shono, K., Borelli, S., Hillbrand, A. & Conigliaro, M., eds. 2017. Agroforestry in rice-production landscapes in Southeast Asia: a practical manual. Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific, Bangkok, Thailand & World Agroforestry Centre (ICRAF) Southeast Asia Regional Program, Bogor, Indonesia. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) or of ICRAF The World Agroforestry Centre concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

I TREE ATTRIBUTE RANKING and PHENOLOGY STUDY: FARMERS
TREE ATTRIBUTE RANKING AND PHENOLOGY STUDY: FARMERS’ KNOWLEDGE OF TREES COMMONLY FOUND ON COFFEE FARMS BORDERING MABIRA FOREST RESERVE IN MUKONO DISTRICT, UGANDA BY MUCHELO RONALD W.OMELI AG332-0855/2009 A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR AWARD OF THE DEGREE OF MASTER OF SCIENCE IN RESAERCH METHODS OF JOMO KENYATTA UNIVERSITY OF AGRICULTURE AND TECHNOLOGY September 2011 i Declaration This dissertation is my original work and has not been presented for a degree in any other University Sign: ______________________________ Date: ______________________ Muchelo Ronald Omeli Recommendation This dissertation has been submitted for examination with our approval as the supervisors of the candidate Sign: ______________________________ Date: ______________________ Dr. Samuel Mwalili JKUAT, Kenya Sign: ______________________________ Date: ______________________ Prof. Catherine Muthuri JKUAT, Kenya Sign: ______________________________ Date: ______________________ Prof. Fergus Sinclair ICRAF, Nairobi ii Dedication This dissertation is dedicated to my sisters; Rachel and Sylvia iii Acknowledgment Great appreciation goes to RUFORUM for all the financial support through the scholarship and JKUAT for delivering the course. Special appreciation also goes to Professor Fergus Sinclair and Dr. Anja Gassner for allowing me to be part of their research team and continued technical support. Dr. Mwalili and Prof Muthuri in the same measure are greatly acknowledged. Thanks a lot for sparing your valuable time trying to make sure I am on the right track with this research. Your guidance and constant follow up of my progress has enabled me to make this progress. Appreciation also goes to Genevieve Lamond for and Emilie Smith for their assistance and guidance. Finally I would to extend my appreciation to, NAFORI, ICRAF and CAFNET project team for all the assistance they offered me during my entire time at the institution. -

Check List Lists of Species Check List 11(4): 1718, 22 August 2015 Doi: ISSN 1809-127X © 2015 Check List and Authors
11 4 1718 the journal of biodiversity data 22 August 2015 Check List LISTS OF SPECIES Check List 11(4): 1718, 22 August 2015 doi: http://dx.doi.org/10.15560/11.4.1718 ISSN 1809-127X © 2015 Check List and Authors Tree species of the Himalayan Terai region of Uttar Pradesh, India: a checklist Omesh Bajpai1, 2, Anoop Kumar1, Awadhesh Kumar Srivastava1, Arun Kumar Kushwaha1, Jitendra Pandey2 and Lal Babu Chaudhary1* 1 Plant Diversity, Systematics and Herbarium Division, CSIR-National Botanical Research Institute, 226 001, Lucknow, India 2 Centre of Advanced Study in Botany, Banaras Hindu University, 221 005, Varanasi, India * Corresponding author. E-mail: [email protected] Abstract: The study catalogues a sum of 278 tree species and management, the proper assessment of the diversity belonging to 185 genera and 57 families from the Terai of tree species are highly needed (Chaudhary et al. 2014). region of Uttar Pradesh. The family Fabaceae has been The information on phenology, uses, native origin, and found to exhibit the highest generic and species diversity vegetation type of the tree species provide more scope of with 23 genera and 44 species. The genus Ficus of Mora- such type of assessment study in the field of sustainable ceae has been observed the largest with 15 species. About management, conservation strategies and climate change 50% species exhibit deciduous nature in the forest. Out etc. In the present study, the Terai region of Uttar Pradesh of total species occurring in the region, about 63% are has been selected for the assessment of tree species as it native to India. -

FLAVONOIDS from Albizia Chinensis of EGYPT
FLAVONOIDS FROM Albizia chinensis OF EGYPT NEVEEN S. GHALY1, F. R. MELEK1* , NAYERA A. M. ABDELWAHED2 (Received August 2010; Accepted December 2010) ABSTRACT The flavonoids kaempferol-3-O-α-L-rhamnopyranoside, quercetin-3-O-α-L-rham- nopyranoside together with luteolin, kaempferol and quercetin, were isolated from the methanolic extract of the leaves of Albizia chinensis collected from Egypt. Iden- tification of the flavonoid constituents was carried by analysing their spectroscopic data and/or by comparing these data with those reported in the literature. The first three isolates were tested for their antimicrobial activity and the results revealed that the tested compounds exhibited moderate inhibiting activity against gram- positive and gram-negative bacteria while no antifungal activity was observed. Key words: Albizia chinensis, Leguminosa, flavonoids, antimicrobial activity RESUMEN Se llevo a cabo el aislamiento de los flavonoides Kaemferol-3-O- α-L-ramnopirano- sida, quercetina-3-O-α-L-ramnopiranosida, luteolina, kaemferol y quercetina, del extracto metanolico de las hojas de Albizia chinensis colectada de Egipto. La iden- tificación de los compuestos se llevo a cabo mediante el análisis espectroscópico. Se valoro la actividad antimicrobiana de los tres primeros compuestos mostrando actividad moderada contra bacterias gram-positivas y gram-negativas. No se obs- ervo actividad antifungicida de estos compuestos. Palabras clave: Albizia chinensis, Leguminosa, flavonoides, actividad antimicro- biana INTRODUCTION wounds and as anthelmintic (Watt and Breyer-brandwijk, 1962). In traditional The genus Albizia (Leguminosa) comprises Chinese medicine, Albizia members are about 150 species widely distributed in used for the treatment of insominia, irri- Africa and Central and South America. In tability, wounds and as antidysentric, Africa, several Albizia species are used in antiseptic and antitubercular (Chadha, folk medicine for the treatment of rheuma- 1985). -

Phytochemical Analysis of Albizia Chinensis (Osbeck)Merr Medicinal Plant
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN:2278-3008, p-ISSN:2319-7676. Volume 12, Issue 6 Ver. I (Nov. – Dec. 2017), PP 89-92 www.iosrjournals.org Phytochemical Analysis Of Albizia Chinensis (Osbeck)Merr Medicinal Plant Amudha. P1., Prabakaran, R2., Senthil Kumar, S1., Gopinath, L. R3 1 Department of Botany, Vivekanandha College of Arts and Sciences for Women, Namakkal, Tamilnadu, India. 2 Department of Botany, Ramakrishna mission Vivekananda College (Autonomous) Chennai. 3 Department of Biotechnology, Vivekanandha Educational Institutions, Namakkal, Tamilnadu, India. Abstract: The frond of Albizia chinensis(osbeck)merr extract was obtained from the powder using 80% methanol. Preliminary phytochemical works were carried out for detection of secondary metabolite. Estimation of alkaloids, flavonoids, protein, saponins, was carried out using appropriate test. A thin layer chromatographic technique was used for compound separation and identification from the extract. The total ash, acid insoluble ash, sulphate ash, water soluble ash values of entire plants of control and commercial raw drugs have been revealed. The control of total ash values of entire plant Albizia chinensis is 25mg, 23.19mg, 63mg, 0.52mg, and 65.5mg. The fluorescence character of entire plant Albizia chinensis was undergone in day light and UV 254nm. The extract was subjected to GC-MS analysis and 10 compounds shown use activities. 10 therapeutically active compounds present in the extract. This compound identification could provide valuable information -

Tree Resources of Katerniaghat Wildlife Sanctuary, Uttar Pradesh, India with Especial Emphasis on Conservation Status, Phenology and Economic Values
INTERNATIONAL JOURNAL OF ENVIRONMENT Volume-3, Issue-1, Dec-Feb 2013/14 ISSN 2091-2854 Received: 10 January Revised: 17 January Accepted: 21 January TREE RESOURCES OF KATERNIAGHAT WILDLIFE SANCTUARY, UTTAR PRADESH, INDIA WITH ESPECIAL EMPHASIS ON CONSERVATION STATUS, PHENOLOGY AND ECONOMIC VALUES Lal Babu Chaudhary1*, Anoop Kumar2, Ashish K. Mishra3, Nayan Sahu4, Jitendra Pandey5, Soumit K. Behera6 and Omesh Bajpai7 1,2,3,4,6,7Plant Diversity, Systematics and Herbarium Division, CSIR-National Botanical Research Institute, Rana Pratap Marg, Lucknow, Uttar Pradesh-226 001, India 5,7Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, Uttar Pradesh- 221 005, India *Corresponding author: [email protected] Abstract Uttar Pradesh, one of the most populated states of India along international border of Nepal, contributes only about 3% of total forest & tree cover of the country as the major parts of the area is covered by agriculture lands and human populations. The forests are quite fragmented and facing severe anthropogenic pressure in many parts. To protect the existing biodiversity, several forest covers have been declared as National Parks and Wildlife Sanctuaries. In the present study, Katerniaghat Wildlife Sanctuary (KWS) has been selected to assess tree diversity, their phenology and economic values as the trees are the major constituent of any forest and more fascinating among all plant groups. The sanctuary consists of tropical moist deciduous type of vegetation and situated along the Indo-Nepal boarder in Bahraich district of Uttar Pradesh, India. After, thorough assessment of the area, a list of 141 tree species belonging to 101 genera and 38 families have been prepared. -

Mineral Nutrient Disorders of Root Crops in the Pacific
Mineral Nutrient Disorders of Root Crops in the Pacific Proceedings of a workshop, Nuku'alofa, Kingdom of Tonga, 17-20 April 1995 Editors: E.T. Craswell, C.J. Asher and J.N. Q'Sullivan Hosted by: Ministry of Agriculture and Forestry, Kingdom of Tonga Sponsored by: South Pacific Commission Pacific Regional Agricultural Research Programme Australian Centre for International Agricultural Research Australian Agency for International Development University of Queensland The Australian Centre for International Agricultural Researeh (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its mandate is to help identify agricultural problems in developing eountries and to commission collaborative research between Australian and developing country researchers in fields where Australia has special research competence. Where trade names are used this does not constitute endorsement of nor discrimination against any product by the Centre. ACIAR PROCEEDINGS This series of publications includes the full proceedings of research workshops or symposia organised or supported by ACIAR. Numbers in this series are distributed internationally to selected individuals and scientific institutions. © Australian Centre for International Agricultural Research, a.p.c. Box 1571, Canberra, ACT 2601 Australia Craswell, E.T., Asher, C.l and O'Sullivan, 1.N. 1996. Mineral Nutrient Disorders of Root Crops in the Pacific. Proceedings of a workshop, Nuku'alofa, Kingdom of Tonga, 17-20 April 1995. ACIAR Proceedings No. 65,142 pp. ISBN I 86320 170 X Editorial management: P. W. Lynch Typesetting and page layout: Sun Photoset Pty Ltd, Brisbane, Australia Printed by: Watson Ferguson & Co., Brisbane, Australia Contents Research, development and extension needs for overcoming nutritional limitations of root crops in the Pacific: a Workshop Summary J.N. -

Studies on the Diversity of Selected Group of Insects in the Parambikulam Wildlife Sanctuary
KFRI Research Report 165 STUDIES ON THE DIVERSITY OF SELECTED GROUP OF INSECTS IN THE PARAMBIKULAM WILDLIFE SANCTUARY V.V. Sudheendrakumar George Mathew KERALA FOREST RESEARCH INSTITUTE PEECHI, THRISSUR March 1999 Pages: 77 CONTENTS Page File Abstract ii r.165.2 1 Introduction 1 r.165.3 2 Study Site 5 r.165.4 3 Materials and Methods 10 r.165.5 4 Results and Discussion 14 r.165.6 5 General Discussion 47 r.165.7 6 References 52 r.165.8 7 Appendix 55 r.165.9 The study was carried out in the Parambikulam Wildlife Sanctuary, in Kerala. during 1994-97 with an objective to prepare an inventory of insects belonging to Hymenoptera (limited to macro forms) and Lepidoptera in the different forest types and to estimate the insect diversity. Altogether 1049 species of insects belonging to 13 orders and 106 families were collected from the study area. Of these 636 species belonging to 13 orders and 106 families were identified. Out of the 484 species of lepidopterans collected, 401 species belonging to 31 families were identifed. which include 124 butterflies and 277 moths. Among the 105 species of macro hymenopterans collected 84 species belonging to 16 families were identified. The overall diversity index for the study area was 4.763. The overall insect diversity index was significantly higher in the moist deciduous forest (4.835) than that in the teak plantations (4.318). The diversity index for evergreen based on ten months data was 4.509. The overall diversity index varied significantly between years in the study area.