Ep 1 803 730 A1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TESS Discovery of a Super-Earth and Three Sub-Neptunes Hosted by the Bright, Sun-Like Star HD 108236
Swarthmore College Works Physics & Astronomy Faculty Works Physics & Astronomy 2-1-2021 TESS Discovery Of A Super-Earth And Three Sub-Neptunes Hosted By The Bright, Sun-Like Star HD 108236 T. Daylan K. Pinglé J. Wright M. N. Günther K. G. Stassun Follow this and additional works at: https://works.swarthmore.edu/fac-physics See P nextart of page the forAstr additionalophysics andauthors Astr onomy Commons Let us know how access to these works benefits ouy Recommended Citation T. Daylan, K. Pinglé, J. Wright, M. N. Günther, K. G. Stassun, S. R. Kane, A. Vanderburg, D. Jontof-Hutter, J. E. Rodriguez, A. Shporer, C. X. Huang, T. Mikal-Evans, M. Badenas-Agusti, K. A. Collins, B. V. Rackham, S. N. Quinn, R. Cloutier, K. I. Collins, P. Guerra, Eric L.N. Jensen, J. F. Kielkopf, B. Massey, R. P. Schwarz, D. Charbonneau, J. J. Lissauer, J. M. Irwin, Ö Baştürk, B. Fulton, A. Soubkiou, B. Zouhair, S. B. Howell, C. Ziegler, C. Briceño, N. Law, A. W. Mann, N. Scott, E. Furlan, D. R. Ciardi, R. Matson, C. Hellier, D. R. Anderson, R. P. Butler, J. D. Crane, J. K. Teske, S. A. Shectman, M. H. Kristiansen, I. A. Terentev, H. M. Schwengeler, G. R. Ricker, R. Vanderspek, S. Seager, J. N. Winn, J. M. Jenkins, Z. K. Berta-Thompson, L. G. Bouma, W. Fong, G. Furesz, C. E. Henze, E. H. Morgan, E. Quintana, E. B. Ting, and J. D. Twicken. (2021). "TESS Discovery Of A Super-Earth And Three Sub-Neptunes Hosted By The Bright, Sun-Like Star HD 108236". -

Article Case Report Congenital Hepatic Fibrosis
Article Case report Congenital hepatic fibrosis: case report and review of literature Brahim El Hasbaoui, Zainab Rifai, Salahiddine Saghir, Anas Ayad, Najat Lamalmi, Rachid Abilkassem, Aomar Agadr Corresponding author: Zainab Rifai, Department of Pediatrics, Children’s Hospital, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, Morocco. [email protected] Received: 19 Jan 2021 - Accepted: 03 Feb 2021 - Published: 18 Feb 2021 Keywords: Fibrosis, hyper-transaminasemia, cholestasis, ciliopathy, case report Copyright: Brahim El Hasbaoui et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Cite this article: Brahim El Hasbaoui et al. Congenital hepatic fibrosis: case report and review of literature. Pan African Medical Journal. 2021;38(188). 10.11604/pamj.2021.38.188.27941 Available online at: https://www.panafrican-med-journal.com//content/article/38/188/full Congenital hepatic fibrosis: case report and review &Corresponding author of literature Zainab Rifai, Department of Pediatrics, Children’s Hospital, Faculty of Medicine and Pharmacy, Brahim El Hasbaoui1, Zainab Rifai2,&, Salahiddine University Mohammed V, Rabat, Morocco Saghir1, Anas Ayad1, Najat Lamalmi3, Rachid 1 1 Abilkassem , Aomar Agadr 1Department of Pediatrics, Military Teaching Hospital Mohammed V, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, Morocco, 2Department of Pediatrics, Children’s Hospital, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, Morocco, 3Department of Histopathologic, Avicenne Hospital, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, Morocco Article characterized histologically by defective Abstract remodeling of the ductal plate (DPM). -
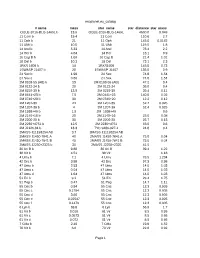
Exoplanet.Eu Catalog Page 1 # Name Mass Star Name
exoplanet.eu_catalog # name mass star_name star_distance star_mass OGLE-2016-BLG-1469L b 13.6 OGLE-2016-BLG-1469L 4500.0 0.048 11 Com b 19.4 11 Com 110.6 2.7 11 Oph b 21 11 Oph 145.0 0.0162 11 UMi b 10.5 11 UMi 119.5 1.8 14 And b 5.33 14 And 76.4 2.2 14 Her b 4.64 14 Her 18.1 0.9 16 Cyg B b 1.68 16 Cyg B 21.4 1.01 18 Del b 10.3 18 Del 73.1 2.3 1RXS 1609 b 14 1RXS1609 145.0 0.73 1SWASP J1407 b 20 1SWASP J1407 133.0 0.9 24 Sex b 1.99 24 Sex 74.8 1.54 24 Sex c 0.86 24 Sex 74.8 1.54 2M 0103-55 (AB) b 13 2M 0103-55 (AB) 47.2 0.4 2M 0122-24 b 20 2M 0122-24 36.0 0.4 2M 0219-39 b 13.9 2M 0219-39 39.4 0.11 2M 0441+23 b 7.5 2M 0441+23 140.0 0.02 2M 0746+20 b 30 2M 0746+20 12.2 0.12 2M 1207-39 24 2M 1207-39 52.4 0.025 2M 1207-39 b 4 2M 1207-39 52.4 0.025 2M 1938+46 b 1.9 2M 1938+46 0.6 2M 2140+16 b 20 2M 2140+16 25.0 0.08 2M 2206-20 b 30 2M 2206-20 26.7 0.13 2M 2236+4751 b 12.5 2M 2236+4751 63.0 0.6 2M J2126-81 b 13.3 TYC 9486-927-1 24.8 0.4 2MASS J11193254 AB 3.7 2MASS J11193254 AB 2MASS J1450-7841 A 40 2MASS J1450-7841 A 75.0 0.04 2MASS J1450-7841 B 40 2MASS J1450-7841 B 75.0 0.04 2MASS J2250+2325 b 30 2MASS J2250+2325 41.5 30 Ari B b 9.88 30 Ari B 39.4 1.22 38 Vir b 4.51 38 Vir 1.18 4 Uma b 7.1 4 Uma 78.5 1.234 42 Dra b 3.88 42 Dra 97.3 0.98 47 Uma b 2.53 47 Uma 14.0 1.03 47 Uma c 0.54 47 Uma 14.0 1.03 47 Uma d 1.64 47 Uma 14.0 1.03 51 Eri b 9.1 51 Eri 29.4 1.75 51 Peg b 0.47 51 Peg 14.7 1.11 55 Cnc b 0.84 55 Cnc 12.3 0.905 55 Cnc c 0.1784 55 Cnc 12.3 0.905 55 Cnc d 3.86 55 Cnc 12.3 0.905 55 Cnc e 0.02547 55 Cnc 12.3 0.905 55 Cnc f 0.1479 55 -

Exoplanet.Eu Catalog Page 1 Star Distance Star Name Star Mass
exoplanet.eu_catalog star_distance star_name star_mass Planet name mass 1.3 Proxima Centauri 0.120 Proxima Cen b 0.004 1.3 alpha Cen B 0.934 alf Cen B b 0.004 2.3 WISE 0855-0714 WISE 0855-0714 6.000 2.6 Lalande 21185 0.460 Lalande 21185 b 0.012 3.2 eps Eridani 0.830 eps Eridani b 3.090 3.4 Ross 128 0.168 Ross 128 b 0.004 3.6 GJ 15 A 0.375 GJ 15 A b 0.017 3.6 YZ Cet 0.130 YZ Cet d 0.004 3.6 YZ Cet 0.130 YZ Cet c 0.003 3.6 YZ Cet 0.130 YZ Cet b 0.002 3.6 eps Ind A 0.762 eps Ind A b 2.710 3.7 tau Cet 0.783 tau Cet e 0.012 3.7 tau Cet 0.783 tau Cet f 0.012 3.7 tau Cet 0.783 tau Cet h 0.006 3.7 tau Cet 0.783 tau Cet g 0.006 3.8 GJ 273 0.290 GJ 273 b 0.009 3.8 GJ 273 0.290 GJ 273 c 0.004 3.9 Kapteyn's 0.281 Kapteyn's c 0.022 3.9 Kapteyn's 0.281 Kapteyn's b 0.015 4.3 Wolf 1061 0.250 Wolf 1061 d 0.024 4.3 Wolf 1061 0.250 Wolf 1061 c 0.011 4.3 Wolf 1061 0.250 Wolf 1061 b 0.006 4.5 GJ 687 0.413 GJ 687 b 0.058 4.5 GJ 674 0.350 GJ 674 b 0.040 4.7 GJ 876 0.334 GJ 876 b 1.938 4.7 GJ 876 0.334 GJ 876 c 0.856 4.7 GJ 876 0.334 GJ 876 e 0.045 4.7 GJ 876 0.334 GJ 876 d 0.022 4.9 GJ 832 0.450 GJ 832 b 0.689 4.9 GJ 832 0.450 GJ 832 c 0.016 5.9 GJ 570 ABC 0.802 GJ 570 D 42.500 6.0 SIMP0136+0933 SIMP0136+0933 12.700 6.1 HD 20794 0.813 HD 20794 e 0.015 6.1 HD 20794 0.813 HD 20794 d 0.011 6.1 HD 20794 0.813 HD 20794 b 0.009 6.2 GJ 581 0.310 GJ 581 b 0.050 6.2 GJ 581 0.310 GJ 581 c 0.017 6.2 GJ 581 0.310 GJ 581 e 0.006 6.5 GJ 625 0.300 GJ 625 b 0.010 6.6 HD 219134 HD 219134 h 0.280 6.6 HD 219134 HD 219134 e 0.200 6.6 HD 219134 HD 219134 d 0.067 6.6 HD 219134 HD -

Fasig-Tipton Fountain of Youth S
reduced 10% Barn 1&2 Hip No. Consigned by Taylor Made Sales Agency, Agent IV 1 Calle Vista Pleasant Colony . His Majesty Pleasantly Perfect . {Sun Colony {Regal State . Affirmed Calle Vista . {La Trinite (FR) Bay mare; Oak Dancer . Green Dancer foaled 2006 { Campagnarde (ARG) . {Oak Hill (1987) {Celine . Lefty {Celestine By PLEASANTLY PERFECT (1998), $7,789,880, Breeders’ Cup Classic [G1], Dubai World Cup [G1], etc. Sire of 8 crops, 14 black type winners, $16,853,945, including Silverside (horse of the year), Shared Account ($1,649,427, Breeders’ Cup Filly & Mare Turf [G1], etc.), Cozi Rosie [G2] ($527,280), Nonios [G3] ($459,000), My Adonis (to 6, 2015, $475,250). 1st dam CAMPAGNARDE (ARG), by Oak Dancer. 3 wins at 2 and 3 in Argentina, champion filly at 3, Gran Premio Seleccion-Argentine Oaks [G1], Premio Bolivia, 2nd Gran Premio Jorge de Atucha [G1], 3rd Copa de Plata [G1], etc.; 4 wins at 4 and 6, $499,225 in N.A./U.S., Ramona H. [G1], Honey Fox H. [L] (DMR, $35,350), Hurry Countess S.-R (SA, $32,000), 3rd Yellow Ribbon Invitational S. [G1], Santa Maria H. [G1], Las Palmas H. [G2], Honey Fox H. [L] (DMR, $8,250), etc. Dam of 15 foals of racing age, including a 2-year-old of 2015, fourteen to race, 10 winners, including-- RIZE (g. by Theatrical-IRE). 16 wins, 3 to 8, $728,680, Phillip H. Iselin H. [G2], Creme Fraiche S. [L] (MED, $45,000), 2nd Oceanport H. [G3], William Donald Schaefer H. [G3], Skip Away S. [L] (MTH, $15,000), 3rd Donn H. -

Exclusively Made Barn 14 Hip No. 1
Consigned by Tres Potrillos, Agent Barn Hip No. 14 Exclusively Made 1 Gone West Elusive Quality.................. Touch of Greatness Exclusive Quality .............. Glitterman Exclusively Made First Glimmer.................... Bay Filly; First Stand March 8, 2011 Danzig Furiously........................... Whirl Series Mad About Julie ............... (1999) Restless Restless Restless Julie.................... Witches Alibhai By EXCLUSIVE QUALITY (2003). Black-type winner of $92,600, Spectacular Bid S. [L] (GP, $45,000). Sire of 3 crops of racing age, 166 foals, 94 start- ers, 59 winners of 116 races and earning $2,727,453, including Sr. Quisqueyano ($319,950, Calder Derby (CRC, $150,350), etc.), Exclusive- ly Maria ($100,208, Cassidy S. (CRC, $60,000), etc.), Boy of Summer ($89,010, Journeyman Stud Sophomore Turf S.-R (TAM, $45,000)), black- type-placed Quality Lass ($158,055), Backstage Magic ($128,540). 1st dam MAD ABOUT JULIE, by Furiously. Winner at 3 and 4, $33,137. Dam of 4 other registered foals, 4 of racing age, 2 to race, 2 winners, including-- Jeongsang Party (f. by Exclusive Quality). Winner at 2, placed at 3, 2013 in Republic of Korea. 2nd dam Restless Julie, by Restless Restless. 2 wins at 2, $30,074, 2nd American Beauty S., etc. Dam of 11 other foals, 10 to race, all winners, including-- RESTING MOTEL (c. by Bates Motel). 8 wins at 2 and 3 in Mexico, cham- pion sprinter, Clasico Beduino, Handicap Cristobal Colon, Clasico Velo- cidad; winner at 4, $46,873, in N.A./U.S. PRESUMED INNOCENT (f. by Shuailaan). 7 wins, 3 to 6, $315,190, Jersey Lilly S. (HOU, $30,000), 2nd A.P. -

NGTS Clusters Survey – III: a Low-Mass Eclipsing Binary in the Blanco 1 Open Cluster Spanning the Fully Convective Boundary
MNRAS 000,1–21 (2021) Preprint 3 September 2021 Compiled using MNRAS LATEX style file v3.0 NGTS clusters survey – III: A low-mass eclipsing binary in the Blanco 1 open cluster spanning the fully convective boundary Gareth D. Smith,1¢ Edward Gillen,2,1y Didier Queloz,1 Lynne A. Hillenbrand,3 Jack S. Acton,4 Dou- glas R. Alves,11 David R. Anderson,5,6 Daniel Bayliss,5,6 Joshua T. Briegal,1 Matthew R. Burleigh,4 Sarah L. Casewell,4 Laetitia Delrez,7,8 Georgina Dransfield,9 Elsa Ducrot,8 Samuel Gill,5,6 Michaël Gillon,8 Michael R. Goad,4 Maximilian N. Günther,10z Beth A. Henderson,4 James S. Jenkins,11,12 Emmanuël Jehin,7 Maximiliano Moyano,13 Catriona A. Murray,1 Peter P. Pedersen,1 Daniel Sebastian,9 Samantha Thompson,1 Rosanna H. Tilbrook,4 Amaury H.M.J. Triaud,9 Jose I. Vines,11 Peter J. Wheatley5,6 1Astrophysics Group, Cavendish Laboratory, J.J. Thomson Avenue, Cambridge CB3 0HE, UK 2Astronomy Unit, Queen Mary University of London, Mile End Road, London E1 4NS, UK 3California Institute of Technology, Department of Astronomy; 1200 East California Blvd; Pasadena, CA, 91125, USA 4School of Physics and Astronomy, University of Leicester, University Road, Leicester, LE1 7RH, UK 5 Department of Physics, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK 6 Centre for Exoplanets and Habitability, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK 7Space sciences, Technologies and Astrophysics Research (STAR) Institute, University of Liège, Belgium 8Astrobiology Research Unit, University of Liège, Allée du 6 août, 19, 4000 Liège (Sart-Timan), Belgium 9School of Physics & Astronomy, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK 10Department of Physics, and Kavli Institute for Astrophysics and Space Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 11Departamento de Astronomia, Universidad de Chile, Casilla 36-D, Santiago, Chile 12Núcleo de Astronomía, Facultad de Ingeniería y Ciencias, Universidad Diego Portales, Av. -

Juliette's CV
Juliette Becker Department of Geological and Planetary Sciences • Caltech • Pasadena, CA 91125 [email protected] • jcbastronomy.com • @jcbastro Research Interests / Goals My major goal in my research is to understand planet formation through studying the boundary conditions of the processes that allow planetary systems to assemble. Appointments 51 Pegasi b Postdoctoral Fellow, Caltech Sept. 2019 - present Postdoctoral Scholar (funded by Leinweber Fellowship), University of Michigan Summer 2019 Education University of Michigan Ann Arbor, MI M.S. in Astronomy and Astrophysics August 2016 PhD in Astronomy and Astrophysics (advisor: Fred Adams) May 2019 California Institute of Technology Pasadena, CA B.S. Astrophysics with honor and a minor in English September 2010 - June 2014 Awards Ralph B. Baldwin Prize in Astronomy 2021 2019 ProQuest Distinguished Dissertation Award 2020 51 Pegasi b Fellowship 2019 Leinweber Center for Theoretical Physics Graduate Fellowship 2018 University of Michigan Aspire, Advance, Achieve Mentoring Award (Nominee) 2018 DPS Bill Hartmann Student Travel Grant 2017 K2SciCon Student Travel Award 2015, 2019 DDA/AAS Raynor L. Duncombe Prize for Student Research 2015 National Science Foundation Graduate Research Fellowship 2014-2019 Chambliss Astronomy Achievement Student Awards, honorable mention 2014 Golden Ankle Dedication and Leadership Award (Caltech) 2011, 2013, 2014 Richter Scholar, Summer Undergraduate Research Fellow (Caltech) 2013 Celia Peterson Leadership Award (Caltech) 2012, 2013 Alain Porter Memorial Summer Undergraduate Research Fellow (Caltech) 2012 ARCS (Achievement Rewards for College Scientists) Fellowship 2012, 2013, 2014 SCIAC Scholar-Athlete Award 2011, 2012, 2013, 2014 Lingle Merit Award (Caltech) 2011 Peer Reviewed Publications 38 total: 11 first author, 8 second author, h-index = 18, total citations ∼ 895 First Author Publications: 38. -

Andrew Vanderburg 77 Massachusetts Avenue • Mcnair Building (MIT Building 37) • Cambridge, MA 02139 [email protected] •
Andrew Vanderburg 77 Massachusetts Avenue • McNair Building (MIT Building 37) • Cambridge, MA 02139 [email protected] • https://avanderburg.github.io Appointments Assistant Professor of Physics at the Massachusetts Institute of Technology July 2021 - present Assistant Professor of Astronomy at The University of Wisconsin-Madison August 2020 - August 2021 Research Associate at the Smithsonian Astrophysical Observatory September 2017 - present NASA Sagan Postdoctoral Fellow at The University of Texas at Austin September 2017 - August 2020 Postdoctoral Associate at Harvard University July 2017 - September 2017 Education Harvard University Cambridge, MA Ph.D. Astronomy and Astrophysics (2017) August 2013 - May 2017 A.M. Astronomy and Astrophysics (2015) University of California, Berkeley Berkeley, CA B.A. Physics and Astrophysics (2013) August 2009 - May 2013 Research Interests • Searching for and studying small planets orbiting other stars • Determining detailed physical properties of terrestrial planets • Learning about the origins and evolution of planetary systems • Testing theories of planetary migration by studying the architecture of planetary systems • Measuring the prevalence of planets in different galactic environments • Developing and using new data analysis techniques in astronomy, including machine learning and deep learning. Awards • 2021 Wisconsin Undergraduate Research Scholars Exceptional Mentorship Award • 2020 Scialog Fellow • 2018 NASA Exceptional Public Achievement Medal • 2017 NASA Sagan Fellow • 2016 Publications of the -

CSC 2019 1St Quarter Report RM Edits
SETI INSTITUTE Activity Report Q1 2019 Image: An artist's impression of GJ 667 Cc, a potentially habitable planet orbiting a red dwarf constituent in a trinary star system. By ESO/L. Calçada - ESO, CC BY 4.0. 1 2 Peer-Reviewed Publications (only in press or published) 1. Abdalla H, Aharonian F, Ait Benkhali F, Anguner EO, Arakawa M, et al., including Huber D (2019). VHE γ-ray discovery and multiwavelength study of the blazar 1ES 2322-409. MNRAS 482, 3011-3022. 2. Abdalla H, Aharonian F, Ait Benkhali F, Anguner EO, Arakawa M, et al., including Huber D (2019). The 2014 TeV γ-Ray Flare of Mrk 501 Seen with H.E.S.S.: Temporal and Spectral Constraints on Lorentz Invariance Violation. Astrophys. J. 870, id.93, 9pp. 3. Abdalla H, Aharonian F, Ait Benkhali F, Anguner EO, Arakawa M, et al., including Huber D (2019). Particle transport within the pulsar wind nebula HESS J1825-137. Astron. Astrophys. 621, id.A116, 18pp. 4. Arentoft T, Grundahl F, WhiteTR, Slumstrup D, Handberg R, et al. including Huber D (2019). Asteroseismology of the Hyades red giant and planet host ɛ Tauri⋆. Astron. Astrophys. 622, id.A190, 12pp. 5. Bacalla XL, Linnartz H, Cox NLJ, Cami J, Roueff E, et al. (2019). The EDIBLES survey. IV. Cosmic ray ionization rates in diffuse clouds from near-ultraviolet observations of interstellar OH+. Astron. Astropys. 622, id.A31, 12pp. 6. Baldi, R.D., Rodriguez-Zaurin, J., Chiaberge, M., Capetti, A., Sparks, W.B., McHardy, I.M., 2019, ApJ, 870, 53. Hubble Space Telescope Emission-line Images of Nearby 3CR Radio Galaxies: Two Photoionization, Accretion, and Feedback Modes, astro-ph/1811.04946. -

Vanderbilt University, Department of Physics & Astronomy 6301
CURRICULUM VITAE: KEIVAN GUADALUPE STASSUN Vanderbilt University, Department of Physics & Astronomy 6301 Stevenson Center Ln., Nashville, TN 37235 Phone: 615-322-2828, FAX: 615-343-7263 [email protected] DEGREES EARNED University of Wisconsin—Madison Degree: Ph.D. in Astronomy, 2000 Thesis: Rotation, Accretion, and Circumstellar Disks among Low-Mass Pre-Main-Sequence Stars Advisor: Robert D. Mathieu University of California at Berkeley Degree: A.B. in Physics/Astronomy (double major) with Honors, 1994 Thesis: A Simultaneous Photometric and Spectroscopic Variability Study of Classical T Tauri Stars Advisor: Gibor Basri EMPLOYMENT HISTORY Vanderbilt University Founder and Director, Frist Center for Autism & Innovation, 2018-present Professor of Computer Science, School of Engineering, 2018-present Stevenson Endowed Professor of Physics & Astronomy, College of Arts & Science, 2016-present Senior Associate Dean for Graduate Education & Research, College of Arts & Science, 2015-18 Harvie Branscomb Distinguished Professor, 2015-16 Professor of Physics and Astronomy, 2011-16 Director, Vanderbilt Initiative in Data-intensive Astrophysics (VIDA), 2007-present Founder and Director, Fisk-Vanderbilt Masters-to-PhD Bridge Program, 2004-15 Associate Professor of Physics and Astronomy, 2008-11 Assistant Professor of Physics and Astronomy, 2003-08 Fisk University Adjoint Professor of Physics, 2006-present University of Wisconsin—Madison NASA Hubble Postdoctoral Research Fellow, Astronomy, 2001-03 Area: Observational Studies of Low-Mass Star -

Conference 1 C O N F E R E N C E 16 29 January 2020 18 August, 2021 Dr
Joint Pathology CeJointnter Pathology Center Veterinary PatholVeterinaryogy Service Pathologys Services WEDNESDAY SLIDE CONFERENCE 2021-2022 WEDNESDAY SLIDE CONFER ENCE 2019-2020 Conference 1 C o n f e r e n c e 16 29 January 2020 18 August, 2021 Dr. Ingeborg Langohr, DVM, PhD, DACVP Professor Department of Pathobiological Sciences Louisiana S tate University School of Veterinary Medicine Baton RouJointge, L APathology Center Silver Spring, Maryland CASE I: CASE S1809996 1: 1 ((4152313JPC 4135077-00) ). Microscoptan/purple.ic Descr ipThetio n:medullary The inte rstitparenchymaium was within thedisrupted section isby diff dozensusel yof infilt brightra tedred btoy dull purple SignalmeSignalment:nt: A 3-mont h-old, male, mixed- moderate linesto lar thatge radiatednumbers from of pre thedomi levelna ofntl they renal crest breed pig 6(-Susmonth scrofa-old )male (intact) mastiff (Canis mononuclethroughar cell thes a lonmedulla.g with edema. There familiaris). is abunda nt type II pneumocyte hyperplasia History: This pig had no previous signs of lining alveolar septae and many of the illness, andHistory: was found dead. The patient was referred to a veterinary medicalalveola r spaces have central areas of Gross Patholteachingogy : hospitalApprox imfollowingately 70% an o facute, 1-dayne- crotic macrophages admixed with other the lungs,history primar ofily lethargy in the c andrani ainappetencel regions o fthat quicklymononucle ar cells and fewer neutrophils. the lobes, progressedwere patch toy obtundation. dark red, and Bloodwork firm performedOc casionally there is free nuclear basophilic comparedat to thethe rDVMmore norevealedrmal ar etooas ofhigh lun tog. read ALT, elevated ALP (805 U/L), hypoalbuminemia (2.7 Laboratorg/dL)y re suandlts: hypoglycemia Porcine reprodu (23 cmg/dL).tive PT and and respiraaPTTtory weresyndrome both prolonged (PRRS) PCat >100R wa secs and >300 positive frsec,om sprespectively.lenic tissue, Protein and P RandRS IHCbilirubin were was strongdetectedly immunore on a urineactive dipstick.