Water Quality and Hydrogeochemical Characteristics of Groundwater Around Mt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatial Uncertainties in Fluoride Levels and Health Risks in Endemic Fluorotic
Groundwater for Sustainable Development 14 (2021) 100618 Contents lists available at ScienceDirect Groundwater for Sustainable Development journal homepage: www.elsevier.com/locate/gsd Research paper Spatial uncertainties in fluoridelevels and health risks in endemic fluorotic regions of northern Tanzania Julian Ijumulana a,b,c,*, Fanuel Ligate a,b,d, Regina Irunde a,b,e, Prosun Bhattacharya a,g, Jyoti Prakash Maity f, Arslan Ahmad g,h,i, Felix Mtalo b a KTH-International Groundwater Arsenic Research Group, Department of Sustainable Development, Environmental Science and Engineering, KTH Royal Institute of Technology, Teknikringen 10B, SE-100 44 Stockholm, Sweden b Department of Water Resources Engineering, College of Engineering and Technology, University of Dar Es Salaam, Dar Es Salaam, Tanzania c Department of Transportation and Geotechnical Engineering, College of Engineering and Technology, University of Dar Es Salaam, Dar Es Salaam, Tanzania d Department of Chemistry, Mkwawa College of Education, University of Dar Es Salaam, Tanzania e Department of Chemistry, College of Natural and Applied Sciences, University of Dar Es Salaam, Tanzania f Department of Earth and Environmental Sciences, National Chung Cheng University, 168 University Road, Min-Hsiung, Chiayi County, 62102, Taiwan g KWR Water Cycle Research Institute, Groningenhaven 7, 3433 PE Nieuwegein, The Netherlands h SIBELCO Ankerpoort NV, Op de Bos 300, 6223 EP Maastricht, the Netherlands i Department of Environmental Technology, Wageningen University and Research (WUR), Wageningen, The Netherlands ARTICLE INFO ABSTRACT Keywords: Spatial uncertainty caused by large-scale variation in fluoride(F ) occurrence remains a setback for water supply Groundwater authorities in the F belts of the world. It is estimated that approximately 80 million people in the East African Fluoride contamination Rift Valley (EARV) regions and volcanic areas exhibit a wide variety of fluorosissymptoms due to drinking water Probability kriging with F‾ concentrations higher than 1.5 mg/L (WHO guideline limit). -

The Copyright of This Thesis Vests in the Author. No Quotation from It Or Information Derived from It Is to Be Published Without Full Acknowledgement of the Source
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town i ESTABLISHMENT OF A COMPREHENSIVE SURVEILLANCE SYSTEM FOR ACUTE PESTICIDE POISONING IN TANZANIA Elikana Eliona Lekei LKXELI001 SUBMITTED TO THE UNIVERSITY OF CAPE TOWN In fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Faculty Health Sciences UNIVERSITY OF CAPE TOWN Date of submission: 27th August 2012 Supervisor: Prof. Leslie London School ofy Public of Health Cape & Family Medicine Town Faculty of Health Sciences Anzio Road, Observatory 7925 South Africa E mail: [email protected] Tel: 27 21 4066524 Universit Co Supervisor: Dr Aiwerasia V Ngowi Muhimbili University of Health and Allied Sciences (MUHAS) School of Public Health and Social Sciences Department of Environmental and Occupational Health P O Box 65015 Dar es Salaam, Tanzania Tel: 255 2150302-6 Ext. 236; Fax: 255 22 2150465 i ii DECLARATION I, Elikana Eliona Lekei, hereby declare that the work on which this dissertation/thesis is based is my original work (except where acknowledgements indicate otherwise) and that neither the whole work nor any part of it has been, is being, or is to be submitted for another degree in this or any other university. I empower the university to reproduce for the purpose of research either the whole or any portion of the contents in any manner whatsoever. -

Arumeru District Catherine W
Conservation agriculture as practised in Tanzania Conservation agriculture in Africa series Series editors Bernard Triomphe Josef Kienzle Martin Bwalya Soren Damgaard-Larsen Titles Conservation agriculture as practised in Ghana Philip Boahen, Benjamin Addo Dartey, Genevieve Delali Dogbe, E. Asare Boadi, Bernard Triomphe, Soren Daamgard-Larsen, John Ashburner Conservation agriculture: a Uganda case study Paul Nyende, Anthony Nyakuni, John Peter Opio, Wilfred Odogola Conservation agriculture in Zambia: a case study of Southern Province Frédéric Baudron, Herbert M. Mwanza, Bernard Triomphe, Martin Bwalya Conservation agriculture as practised in Kenya: two case studies Pascal Kaumbutho, Josef Kienzle, editors Laikipia District Tom Apina, Paul Wamai, Philip Mwangi Siaya District Philip K. Mwangi, Kennedy O. Okelo, Tom Apina Conservation agriculture as practised in Tanzania: three case studies Richard Shetto, Marietha Owenya, editors Arumeru District Catherine W. Maguzu, Dominick E. Ringo, Wilfred Mariki, Marietha Owenya, Flora Kola, Charles Leseyo Karatu District Dominick E. Ringo, Catherine W. Maguzu, Wilfred Mariki, Marietha Owenya, Njumbo, Frank Swai Mbeya District Saidi Mkomwa, Ahaz Mussei, Remmy Mwakimbwala, Ndabhemeye Mulengera, Elimpaa Kiranga Conservation agriculture as practised in Tanzania: three case studies Richard Shetto, Marietha Owenya, editors Arumeru District Karatu District Mbeya District Publishers African Conservation Tillage Network (ACT) PO Box 14733, Westlands Nairobi 00800, Kenya tel and fax: +254 20 445 1391 website: -
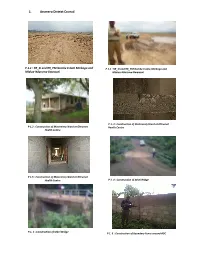
1. Arumeru District Council
1. Arumeru District Council P.1.1 : RR_SI and RR_PM Gombe Estate Mmbogo and P 1.1 : RR_SI and RR_PM Gombe Estate‐Mmbogo and Mlalua‐Nduruma‐Bwawani Mlalua‐Nduruma‐Bwawani P 1. 3 : Construction of Materninty Ward at Oltrumet P 1.2 : Construction of Materninty Ward at Oltrumet Health Centre Health Centre P 1.5 : Construction of Materninty Ward at Oltrumet Health Centre P 1. 4 : Construction of Sekei Bridge P.1. 5 : Construction of Sekei Bridge P 1. 6 : Construction of Boundary Fence around ADC 2. BAHI DISTRICT COUNCIL P 2.1: Aditors at contractor's site office reviewing P2.2: Uncompacted fill and poor concrete drawings workmanship on Kongogo Dam P 2.3: Un‐cemented Riprap at Kongogo Dam P 2.4: Poorly trimmed borrow pit at Kongogo P 2.5: Auditors checking dimensions compliance at Bahi DC P 2.6: Completed Kigwe Mnadani Drift Servant Quarters P 2.7: Auditors confirming a borehole at Mchito P 2.8: Auditors discussing with village leader at Borehole Mundemu on the way forward - dry borehole 3. Bukoba District Council P. 3.1: Cracks can be seen on the floor and walls of a newly constructed Agricultural Centre at Kikomelo P. 3.2: A newly constructed small bridge P 3.3: Oversize, dirty, sand stone coarse aggregates used for bridges/box culverts along Kalebe- Nyakibimbili road P 3.4: A bridge whose width was increased a day after casting without engineer’s approval along Kalebe- Nyakibimbili road 4. Geita District Council P 4.1: Poorly constructed pavement at the New Geita Bus Stand P 4.2: An auditor and PE Technician carrying out P 4.3: Well constructed headwalls and wingwalls and measurements at the New Geita Bus Stand poorly compacted approaches along Geita Town – Katoma Area Roads P 4.4 Poor workmanship on constructed headwall P 4.5 One of the existing “Bridges” along Sungusira and wing walls along Sungusira – Nyakaduha Road Road 5. -

Effectiveness of Adult Education Programme in Promoting
EFFECTIVENESS OF ADULT EDUCATION PROGRAMME IN PROMOTING DEVELOPMENT AMONG THE MAASAI COMMUNITY IN ARUSHA DISTRICT COUNCIL -TANZANIA Einoth Francis Mollel Thesis Submitted to the Post Graduate Studies in Education in Partial Fulfilment of the Requirements for the award of Master’s Degree in Education Administration and Planning THE CATHOLIC UNIVERSITY OF EASTERN AFRICA 2019 DECLARATION i DEDICATION This thesis is dedicated to Mollel family and to Friederike who have been very dear to me during my academic journey. ii ABSTRACT The purpose of this study was to examine the effectiveness of adult education in the promoting development among Maasai community in Rural District of Arusha, Tanzania. Four research questions guided the study: How does adult education programme promote development among Maasai community in Arusha DC? What is the attitude of the people towards the implementation of adult education programme in promoting development among Maasai community in Arusha district council? What are the challenges facing the implementation of adult education programme in Arusha District council? What suggestions can be put forward to ensure effective implementation of adult education programme to promote development among the Maasai community in Arusha District council? The study was anchored in . Andragoyg theory of adult learning. The study was guided by the Convergent Parallel Mixed Method Design. The target population was all educational officers, adult educators and adult learners in the rural district of Arusha. Purposive and snowball sampling techniques were used to select the study participants. Questionnaires and interview guide were used to collect data. Research instruments were subjected to both content and face validity. Cronbach’s Alpha Coefficient technique was used to test reliability for quantitative data and credibility dependability for qualitative data. -

Enhancing Accessibility and Utilization of Mico Credit
SOUTHERN NEW HAMPSHIRE UNIVERSIT Y & THE OPEN UNIVERSITY O F TANZANIA MASTER OF SCIENCE IN COMMUNITY ECONOMIC DEVELOPMENT (2007) ENHANCING ACCESSIBILITY AND UTILIZATION OF MICRO CREDIT AMONG WOMEN AND YOUTHS I N VALESKA AND MALULA VILLAGES, ARUMERU DISTRICT, ARUSHA REGION. KIHULYA, ROSE JOSEPH SOUTHERN NEW HAMPSHIRE UNIVERSIT Y & THE OPEN UNIVERSITY O F TANZANIA ENHANCING ACCESSIBILITY AND UTILIZATION OF MICRO CREDIT AMONG WOMEN AND YOUTHS IN VALESKA AND MALULA VILLAGES, ARUMERU DISTRICT, ARUSHA REGION. A PROJECT SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN COMMUNITY ECONOMIC DEVELOPMENT I N SOUTHERN NEW HAMPSHIRE UNIVERSITY A T THE OPEN UNIVERSITY O F TANZANIA BY, KIHULYA, ROSE JOSEPH SEPTEMBER 2007 TABLE OF CONTENTS SUPERVISOR(S) CERTIFICATION i STATEMENT OF COPYRIGHT i i DECLARATION ii i DEDICATION i v ABSTRACT v ACKNOWLEDGEMENT. v i LIST OF TABLES vii i LIST OF FIGURES i x LIST OF APPENDICES x ACRONYMS AND ABBREVIATIONS x i EXECUTIVE SUMMARY xi i CHAPTER I. COMMUNITY NEEDS ASSESSMENT 1 1.1. Community profile 1 1.1.1. Profile of the host organization - Business Entrepreneur Support Unit 1 1.2. Community Needs Assessment 4 1.2.1. Community Profile - Arumer u District 4 1.2.2. Geographical location 4 1.2.3. Administrative unit 5 1.2.4. Climate and altitude 5 1.2.5. Population Dynamics 6 1.2.6. Ethnic and religious groups 6 1.2.7. Economic activities 7 1.2.8. Markets 7 1.2.9. Basic infrastructure an d communication 8 1.2.10. Social Services 9 1.2.10.1. -

Final Report
Final Report Baseline Study for Hand in Hand Eastern Africa Program in Northern Tanzania August 2017 Table of Contents Table of Contents.................................................................................................................. 2 List of tables.......................................................................................................................... 3 List of figures ........................................................................................................................ 4 List of Abbreviations.............................................................................................................. 5 Acknowledgement................................................................................................................. 6 Executive Summary .............................................................................................................. 7 1. Introduction.................................................................................................................. 13 1.1 Overview of HiH EA Model.................................................................................... 13 1.2 HiH EA Expansion Plan for Tanzania.................................................................... 14 2. The Baseline Study...................................................................................................... 15 2.1 The Scoping Study................................................................................................ 15 2.1.1 Objectives of the Scoping -

Arusha District Council
ARUSHA DISTRICT COUNCIL INVESTMENT PROFILE DISCLOSE THE POTENTIAL OF ARUSHA DISTRICT COUNCIL February, 2017 FOREWORD I would like to welcome all esteemed investors to explore the irresistible opportunities provided by the Arusha District Council. Arusha District Council was established in 2007, the Council has a vision of to be a leading transformed Council that provides high quality services for Sustainable Development of the Community by 2025. In order to increase competitiveness in attracting investors to our District Council, effort and initiative to identify, expose and promote investment opportunities available in Arusha District Council is going on. We are indeed determined to utilize potential areas owned by the Council, Communities and those own by private developer. In this Investment Profile, we give you opportunities to realize your entrepreneurial ambitions and explore them. We believe in supporting our investors’ aspirations as the Council. As we want to be one among the leading investment avenues in Tanzania. In Arusha District Council investors are favoured with presence of suitable investment climate that will help you capitalize on untapped opportunities in Arusha and Tanzania as a whole. Whereby investment can be done to the following areas of interests include tourism, processing industries, livestock and agricultural sector, beekeeping, sports and recreation centre, modern market, real estate, socio-economic services. Investment climate is characterised by peace and stability, availability of raw materials, market, abundant natural resources, road and transportation network, electricity services and the strategic geographical location will support establishment and success of investments. It is because of the above mention few facts we are proud to say that Arusha District Council is the best investment destination of your choice. -

Progress Report: GMH (#0090): an Integrated Approach to Addressing the Issue of Adolescent Depression In
Progress Report: GMH (#0090): An Integrated Approach to Addressing the Issue of Adolescent Depression in Malawi and Tanzania August 5, 2014 Submitted To: Grand Challenges Canada Submitted By: Farm Radio International Progress against milestones for period ending July 15, 2014 MoUs signed with Ministry Health and Education in Tanzania To date, we have held two stakeholder meetings and one mental health training to secure buy-in from relevant government ministries. Representatives from the regional and district level MoE and MoH were invited to attend the meetings. We have also had one face-to-face meeting with the Director of Mental Health Services in the Ministry of Health in Dar es Salam. Representatives from the ministries have indicated their enthusiasm and support for the project, and their willingness to form a technical advisory group and to sing Memoranda of Understanding for the project. MoUs have been sent to the national MoE and MoH, and are awaiting a reply 35 schools selected for participation in target districts in Tanzania 35 secondary schools have been selected for participation in the program, and we have received clearance to conduct activities by the regional and district level representatives from the Ministry of Education. To date, we have completed a baseline survey of 350 youth from 10 schools, and will survey another 350 youth from an additional 10 schools by the end of August, 2014. Attached (annex 1) is a list of 35 schools selected for participation in the program 2 MOUs signed with radio stations We have selected 2 radio stations in the Arusha region to participate in the communication component of the program in Tanzania. -

Pilot Project Preliminary Final Report (Final Report Will Be Submitted at the End of the AVRDC No Cost Extension for Research Activities in June 2014)
Postharvest Extension Pilot Project: Sub-Saharan Africa Barrett/Kitinoja 2013 USAID Horticulture Collaborative Research Support Program (Horticulture CRSP) Pilot Project Preliminary Final Report (Final report will be submitted at the end of the AVRDC no cost extension for research activities in June 2014) Project Title: Extension of Appropriate Postharvest Technology in Sub-Saharan Africa: A Postharvest Training and Services Center October 2013 Lead PI: Diane Barrett Lead University: University of California, Davis (UCD); [email protected] Co-PIs: Lisa Kitinoja, World Food Logistics Organization (WFLO) Jinru Chen, University of Georgia Ngoni Nenguwo, AVRDC –Regional Center for Africa 1 Postharvest Extension Pilot Project: Sub-Saharan Africa Barrett/Kitinoja 2013 Introduction Physical losses of horticultural crops postharvest continue to range from 30-80% in Sub-Saharan Africa (SSA), and problems with food quality, safety and nutritional value have been well documented in recent studies by icipe (Kenya), IDRC, the World Resources Institute and UNFAO. While more than a dozen past international projects have identified appropriate postharvest technologies and recommended a variety of training, capacity building and small- scale infrastructure development (Kitinoja 2010), this was the first project to integrate all of this technology and information and offer a locally based solution. This unique pilot project for smallholder farmers in Arusha, Tanzania combined a wide variety of training programs, adaptive research and demonstrations of postharvest practices and services aimed at reducing losses and increasing shelf life. Via a postharvest shop set up nearby in Njiro, it will provide on-site ready access to the tools and supplies people need in order to reduce postharvest losses and improve market access and incomes for the smallholder farmers, women farmers and village level processors in the northern zone of Tanzania who are affiliated with established cooperatives and farmers associations near Arusha. -

Agwater Solutions Project Case Study
AgWater Solutions Project Case Study Adoption and Outscaling of Conservation Agriculture in Tanzania S.D. Tumbo Soil Water Management Research Group, Sokoine University of Agriculture, Tanzania K.D. Mutabazi Soil Water Management Research Group, Sokoine University of Agriculture, Tanzania F.C. Kahimba Soil Water Management Research Group, Sokoine University of Agriculture, Tanzania W.B. Mbungu Soil Water Management Research Group, Sokoine University of Agriculture, Tanzania September, 2012 Acknowledgment The authors and project partners wish to thank the Bill & Melinda Gates Foundation for the generous grant that made this project possible. The AWM Project The AgWater Solutions project was implemented in five countries in Africa and two states in India between 2008 and 2012. The objective of the project was to identify investment options and opportunities in agricultural water management with the greatest potential to improve incomes and food security for poor farmers, and to develop tools and recommendations for stakeholders in the sector including policymakers, investors, NGOs and small-scale farmers. The leading implementing institutions were the International Water Management Institute (IWMI), the Stockholm Environment Institute (SEI), the Food and Agriculture Organization of the United Nations (FAO), the International Food Policy Research Institute (IFPRI), International Development Enterprises (iDE) and CH2MHill. For more information on the project or detailed reports please visit the project website http://awm-solutions.iwmi.org/home-page.aspx . Disclaimer This report is based on research funded by the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the project, its partners or the Bill & Melinda Gates Foundation. -

A Case of Mount Meru Forest Reserve in Northern Tanzania
FOREST RESOURCE MANAGEMENT FOR SUSTAINABLE DEVELOPMENT: A CASE OF MOUNT MERU FOREST RESERVE IN NORTHERN TANZANIA. By Ufoo C. Lema University of Cape Town A DISSERTATION SUBMITTED TO THE DEPARTMENT OF ENVIRONMENTAL AND GEOGRAPHICAL SCIENCE, UNIVERSITY OF CAPE TOWN, IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF PHILOSOPHY. September, 1997. ~;~}ii::.''..;:_j,;l ttf:{f fl L.::-__ .. ~,_ ...::;~-- -::...:~~ ... .....-.:w. .... -~--:.,~~-.....-,',l,;~'.... .. ,._,s._~-~~-2~~·.-. -~-~ .........". ~.,. The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town ~'t)&f ~O \ , 5 LE- M fr · q<i l ;2'18 ( v Abstract Management systems as applied to most of the world's tropical forests are anthropocentric. In view of this, conservation of these ecosystems has been threatened by overuse and pressure of exotic monocultures. Sustainable forest development aims at utilizing the forest resource without unduly degrading the forest ecosystems. This study examines the case of Mount Meru forest reserve, a tropical montane rainforest in northern Tanzania. The main aim of the study is to investigate human induced causes of forest degradation and to propose ways to improve the forest management so as to attain sustainable forest development. The study is based on survey data obtained from the study area through semi-structured interviews conducted with 198 key informats.