Functional Morphometry of the Pterygoid Hamulus. a Comparative Study of Modern and Medieval Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selecting Different Approaches for Palate and Pharynx Surgery
SPECIAL ISSUE 4: INVITED ARTICLE Selecting Different Approaches for Palate and Pharynx Surgery: Palatopharyngeal Arch Staging System Rodolfo Lugo-Saldaña1 , Karina Saldívar-Ponce2 , Irina González-Sáez3 , Daniela Hernández-Sirit4 , Patricia Mireles-García5 ABSTRACT The examination of the anatomical structures involved in the upper airway collapse in patients with the obstructive sleep apnea-hypopnea syndrome (OSAHS) is a key for integrated evaluation of patients. Our proposal is for a noninvasive classification system that guides us about the presence of anatomical differences between the palatopharyngeal muscle (PFM). The functions of the PFM are narrowing the isthmus, descending the palate, and raising the larynx during swallowing; these characteristics give the PFM a special role in the collapse of the lateral pharyngeal wall. Complete knowledge of the anatomy and classification of different variants can guide us to choose the appropriate surgical procedures for the lateral wall collapse. Until now there is not a consensus about description of the trajectory or anatomical variants of the PFM into oropharynx, the distance between both muscles, and the muscle tone. Here we also present the relationship between the lateral wall surgeries currently available (lateral pharyngoplasty by Cahali, expansion sphincteroplasty by Pang, relocation pharyngoplasty by Li, Roman blinds pharyngoplasty by Mantovani, and barbed sutures pharyngoplasty by Vicini) with the proposed classification of the palatopharyngeal arch staging system (PASS). Keywords: -

Morphology and Fracture Effects of the Hamulus Pterygoid: a Literature Review of the Last 49 Years
Latin American Journal of Development, Curitiba, v. 3, n. 1, p. 475-487, jan./feb. 2021. ISSN 2674-9297 Morphology and fracture effects of the hamulus pterygoid: a literature review of the last 49 years Morfología y efectos de fractura del hamulus pterygoid: una revisión de la literatura de los últimos 49 años DOI: 10.46814/lajdv3n1-041 Recebimento dos originais: 30/10/2020 Aceitação para publicação: 23/12/2020 Polyanne Junqueira Silva Andresen Strini PhD, Federal University of Uberlândia - UFU, Uberlândia, MG, Brazil Address: Rio Preto Street, 178, Lídice, Uberlândia - MG Paulinne Junqueira Silva Andresen Strini PhD, Federal University of Uberlândia - UFU, Uberlândia, MG, Brazil Address: Rio Preto Street, 178, Lídice, Uberlândia - MG ABSTRACT The hamulus pterygoid consists in a relevant anatomical structure, important for fixation of several tendons and muscles, keeping the integrity of soft palate and pharynx. A literature review was conducted in order to investigate the morphology and effect of hamulus pterygoid fracture in clinical manifestation and its relationships with other orofacial components. A literature search was conducted, using Pubmed and Bireme data bases, and covering the time period 1970 to 2019. The Key words for the research were hamulus pterygoid, pterygoid fracture and hamulus pterygoid fracture, resulting in 440 articles, being 41 initials selected. Among them, just 31 were included in the analysis and 08 of the articles were not available through our library system or were in volumes before our holdings began. The remaining were excluded when they weren’t in English idiom, or when didn’t talk about morphological, functional or damages in the hamulus pterygoid. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

Parts of the Body 1) Head – Caput, Capitus 2) Skull- Cranium Cephalic- Toward the Skull Caudal- Toward the Tail Rostral- Toward the Nose 3) Collum (Pl
BIO 3330 Advanced Human Cadaver Anatomy Instructor: Dr. Jeff Simpson Department of Biology Metropolitan State College of Denver 1 PARTS OF THE BODY 1) HEAD – CAPUT, CAPITUS 2) SKULL- CRANIUM CEPHALIC- TOWARD THE SKULL CAUDAL- TOWARD THE TAIL ROSTRAL- TOWARD THE NOSE 3) COLLUM (PL. COLLI), CERVIX 4) TRUNK- THORAX, CHEST 5) ABDOMEN- AREA BETWEEN THE DIAPHRAGM AND THE HIP BONES 6) PELVIS- AREA BETWEEN OS COXAS EXTREMITIES -UPPER 1) SHOULDER GIRDLE - SCAPULA, CLAVICLE 2) BRACHIUM - ARM 3) ANTEBRACHIUM -FOREARM 4) CUBITAL FOSSA 6) METACARPALS 7) PHALANGES 2 Lower Extremities Pelvis Os Coxae (2) Inominant Bones Sacrum Coccyx Terms of Position and Direction Anatomical Position Body Erect, head, eyes and toes facing forward. Limbs at side, palms facing forward Anterior-ventral Posterior-dorsal Superficial Deep Internal/external Vertical & horizontal- refer to the body in the standing position Lateral/ medial Superior/inferior Ipsilateral Contralateral Planes of the Body Median-cuts the body into left and right halves Sagittal- parallel to median Frontal (Coronal)- divides the body into front and back halves 3 Horizontal(transverse)- cuts the body into upper and lower portions Positions of the Body Proximal Distal Limbs Radial Ulnar Tibial Fibular Foot Dorsum Plantar Hallicus HAND Dorsum- back of hand Palmar (volar)- palm side Pollicus Index finger Middle finger Ring finger Pinky finger TERMS OF MOVEMENT 1) FLEXION: DECREASE ANGLE BETWEEN TWO BONES OF A JOINT 2) EXTENSION: INCREASE ANGLE BETWEEN TWO BONES OF A JOINT 3) ADDUCTION: TOWARDS MIDLINE -

Sphenoid Bone and Its Sinus — Anatomo-Clinical Review of the Literature Including Application to FESS
FOLIA MEDICA CRACOVIENSIA Vol. LIX, 2, 2019: 45–59 PL ISSN 0015-5616 DOI: 10.24425/fmc.2019.128453 Sphenoid bone and its sinus — anatomo-clinical review of the literature including application to FESS Joanna Jaworek-Troć1, Michał Zarzecki1, Anna Bonczar2, Lourdes N. Kaythampillai1, Bartosz Rutowicz1, Małgorzata Mazur1, Jacenty Urbaniak1, Wojciech Przybycień1, Katarzyna Piątek-Koziej1, Marcin Kuniewicz1, Marcin Lipski1, Wojciech Kowalski3, Janusz Skrzat1, Marios Loukas4, Jerzy Walocha1 1Department of Anatomy, Jagiellonian University Medical College, Kraków, Poland 2K. Gibiński’s University Center of Silesian Medical University, Katowice, Poland 3Medical Offi ces, Kraków, Poland 4Department of Anatomy, St. Georges University, Grenada, West Indies Corresponding author: Jerzy Walocha, MD, PhD Department of Anatomy, Jagiellonian University Medical College ul. Kopernika 12, 31-034 Kraków, Poland Phone: +48 12 422 95 11; E-mail: [email protected] Abstract: Authors paid attention to anatomy and clinical implications which are associated with the variations of the sphenoid sinus. We discuss also anatomical structure of the sphenoid bone implementing clinical application of this bone to diff erent invasive and miniinvasive procedures (i.e. FESS). Key words: sphenoid bone, sphenoid sinus, anatomy, computer tomography, FESS. Introduction Sphenoid sinuses are pneumatic spaces lined with mucosa, located in the body of the sphenoid bone. Th eir morphology is highly variable. Th eir variability concerns: 46 Joanna Jaworek-Troć, Michał Zarzecki, et al. • Size • Shape • Number of septa • Level of pneumatization Th ere is a lack of unequivocal pattern of the sinuses, which could have been supposed as anatomically normal. Sphenoid sinuses neighbor through their walls with important anatomical structures, both nervous and vascular — this neighbourhood and anatomical composition of the sphenoid sinuses are extremely important for the surgery in these regions. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Appendix B: Muscles of the Speech Production Mechanism
Appendix B: Muscles of the Speech Production Mechanism I. MUSCLES OF RESPIRATION A. MUSCLES OF INHALATION (muscles that enlarge the thoracic cavity) 1. Diaphragm Attachments: The diaphragm originates in a number of places: the lower tip of the sternum; the first 3 or 4 lumbar vertebrae and the lower borders and inner surfaces of the cartilages of ribs 7 - 12. All fibers insert into a central tendon (aponeurosis of the diaphragm). Function: Contraction of the diaphragm draws the central tendon down and forward, which enlarges the thoracic cavity vertically. It can also elevate to some extent the lower ribs. The diaphragm separates the thoracic and the abdominal cavities. 2. External Intercostals Attachments: The external intercostals run from the lip on the lower border of each rib inferiorly and medially to the upper border of the rib immediately below. Function: These muscles may have several functions. They serve to strengthen the thoracic wall so that it doesn't bulge between the ribs. They provide a checking action to counteract relaxation pressure. Because of the direction of attachment of their fibers, the external intercostals can raise the thoracic cage for inhalation. 3. Pectoralis Major Attachments: This muscle attaches on the anterior surface of the medial half of the clavicle, the sternum and costal cartilages 1-6 or 7. All fibers come together and insert at the greater tubercle of the humerus. Function: Pectoralis major is primarily an abductor of the arm. It can, however, serve as a supplemental (or compensatory) muscle of inhalation, raising the rib cage and sternum. (In other words, breathing by raising and lowering the arms!) It is mentioned here chiefly because it is encountered in the dissection. -
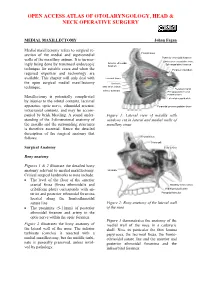
MEDIAL MAXILLECTOMY Johan Fagan
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY MEDIAL MAXILLECTOMY Johan Fagan Medial maxillectomy refers to surgical re- section of the medial and superomedial Frontal sinus walls of the maxillary antrum. It is increas- Posterior ethmoidal foramen Orbital process palatine bone Anterior ethmoidal Sphenopalatine foramen ingly being done by transnasal endoscopic foramen technique for suitable cases and when the Foramen rotundum required expertise and technology are available. This chapter will only deal with Lacrimal fossa the open surgical medial maxillectomy Uncinate Max sinus ostium technique. Pterygoid canal Inferior turbinate Pterygopalatine canal Palatine bone Maxillectomy is potentially complicated Lateral pterygoid plate by injuries to the orbital contents, lacrimal apparatus, optic nerve, ethmoidal arteries, Pyramidal process palatine bone intracranial contents, and may be accom- panied by brisk bleeding. A sound under- Figure 1: Lateral view of maxilla with standing of the 3-dimensional anatomy of windows cut in lateral and medial walls of the maxilla and the surrounding structures maxillary sinus is therefore essential. Hence the detailed description of the surgical anatomy that follows. Frontal sinus Crista galli Surgical Anatomy Sella turcica Bony anatomy Figures 1 & 2 illustrate the detailed bony anatomy relevant to medial maxillectomy. Uncinate Critical surgical landmarks to note include: • The level of the floor of the anterior cranial fossa (fovea ethmoidalis and Maxillary sinus ostium cribriform plate) corresponds with an- Medial pterygoid plate terior and posterior ethmoidal foramina Pterygoid hamulus located along the frontoethmoidal suture line Figure 2: Bony anatomy of the lateral wall • The proximity (5-11mm) of posterior of the nose ethmoidal foramen and artery to the optic nerve within the optic foramen Figure 3 demonstrates the anatomy of the Figure 2 illustrates the bony anatomy of medial wall of the nose in a cadaveric the lateral wall of the nose. -

INFERIOR MAXILLECTOMY Johan Fagan
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY INFERIOR MAXILLECTOMY Johan Fagan Tumours of the hard palate and superior Figure 2 illustrates the bony anatomy of alveolus may be resected by inferior the lateral wall of the nose. The inferior maxillectomy (Figure 1). A Le Fort 1 turbinate (concha) may be resected with osteotomy may also be used as an inferior maxillectomy, but the middle tur- approach to e.g. angiofibromas and the binate is preserved. nasopharynx. Frontal sinus Posterior ethmoidal foramen Orbital process palatine bone Anterior ethmoidal Sphenopalatine foramen foramen Foramen rotundum Lacrimal fossa Uncinate Max sinus ostium Pterygoid canal Inferior turbinate Pterygopalatine canal Palatine bone Lateral pterygoid plate Figure 1: Bilateral inferior maxillectomy Pyramidal process palatine bone A sound understanding of the 3-dimen- Figure 2: Lateral view of maxilla with sional anatomy of the maxilla and the windows cut in lateral and medial walls of surrounding structures is essential to do the maxillary sinus operation safely. Hence the detailed description of the relevant surgical anatomy that follows. Frontal sinus Crista galli Surgical Anatomy Sella turcica Bony anatomy Figures 2, 3 & 4 illustrate the detailed bony anatomy relevant to maxillectomy. Uncinate Critical surgical landmarks to note include: • The floor of the anterior cranial fossa (fovea ethmoidalis and cribriform Maxillary sinus ostium plate) corresponds with anterior and Medial pterygoid plate posterior ethmoidal foramina located, Pterygoid -

Skull / Cranium
Important! 1. Memorizing these pages only does not guarantee the succesfull passing of the midterm test or the semifinal exam. 2. The handout has not been supervised, and I can not guarantee, that these pages are absolutely free from mistakes. If you find any, please, report to me! SKULL / CRANIUM BONES OF THE NEUROCRANIUM (7) Occipital bone (1) Sphenoid bone (1) Temporal bone (2) Frontal bone (1) Parietal bone (2) BONES OF THE VISCEROCRANIUM (15) Ethmoid bone (1) Maxilla (2) Mandible (1) Zygomatic bone (2) Nasal bone (2) Lacrimal bone (2) Inferior nasalis concha (2) Vomer (1) Palatine bone (2) Compiled by: Dr. Czigner Andrea 1 FRONTAL BONE MAIN PARTS: FRONTAL SQUAMA ORBITAL PARTS NASAL PART FRONTAL SQUAMA Parietal margin Sphenoid margin Supraorbital margin External surface Frontal tubercle Temporal surface Superciliary arch Zygomatic process Glabella Supraorbital margin Frontal notch Supraorbital foramen Internal surface Frontal crest Sulcus for superior sagittal sinus Foramen caecum ORBITAL PARTS Ethmoidal notch Cerebral surface impresiones digitatae Orbital surface Fossa for lacrimal gland Trochlear notch / fovea Anterior ethmoidal foramen Posterior ethmoidal foramen NASAL PART nasal spine nasal margin frontal sinus Compiled by: Dr. Czigner Andrea 2 SPHENOID BONE MAIN PARTS: CORPUS / BODY GREATER WINGS LESSER WINGS PTERYGOID PROCESSES CORPUS / BODY Sphenoid sinus Septum of sphenoid sinus Sphenoidal crest Sphenoidal concha Apertura sinus sphenoidalis / Opening of sphenoid sinus Sella turcica Hypophyseal fossa Dorsum sellae Posterior clinoid process Praechiasmatic sulcus Carotid sulcus GREATER WINGS Cerebral surface • Foramen rotundum • Framen ovale • Foramen spinosum Temporal surface Infratemporalis crest Infratemporal surface Orbital surface Maxillary surface LESSER WINGS Anterior clinoid process Superior orbital fissure Optic canal PTERYGOID PROCESSES Lateral plate Medial plate Pterygoid hamulus Pterygoid fossa Pterygoid sulcus Scaphoid fossa Pterygoid notch Pterygoid canal (Vidian canal) Compiled by: Dr. -

Evaluation of the Pterygoid Hamulus Morphology Using Cone Beam Computed Tomography Kaan Orhan, DDS, Phd,A,B Bayram U
Evaluation of the pterygoid hamulus morphology using cone beam computed tomography Kaan Orhan, DDS, PhD,a,b Bayram U. Sakul, PhD,c Ulas Oz, DDS, PhD,d and Burak Bilecenoglu, DDS, PhD,e Ankara and Mersin, Turkey UNIVERSITY OF ANKARA, NEAR EAST UNIVERSITY, ANKARA UNIVERSITY, AND UFUK UNIVERSITY Objective. This study consists of anatomic research of the pterygoid hamulus (PH) using 3D cone beam computed tomography (CBCT) images reconstructed from a volumetric rendering program. Study design. Three hundred ninety-six sides in the CBCT scans of 198 (115 men and 83 women) patients were retrospectively analyzed. DICOM data of the patients were transferred to a surface-rendering software so as to generate 3D hard tissue surface representations of PHs. The width, length, angle, and the distance between posterior nasal spine and tip of the PH were measured. In addition, the inclinations of PHs were also evaluated in sagittal and coronal planes of the 3D images. Pearson 2 and Student t test were performed for statistical analysis among age, localization, and measurements (P Ͻ .05). Results. The mean PH measurements of left and right sides were 1.72 (SD 0.94) and 1.87 (SD 1.17)-mm width, and the lengths were 5.48 (AD 1.94), and 5.40 (SD 2.0) mm, respectively, with no significant difference (P Ͼ .05). All PHs were inclined toward the lateral side in the coronal plane, whereas PHs tended to incline toward the posterior rather than anterior in the sagittal plane (ϳ78%). The results showed no statistically significant differences among age, localization, and measurements of PHs (P Ͼ .05).