Editorial Team
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IJISRT19DEC540 by Ijisrt19dec540 Ijisrt19dec540
IJISRT19DEC540 by Ijisrt19dec540 Ijisrt19dec540 Submission date: 24-Dec-2019 09:53PM (UTC-0800) Submission ID: 1238307746 File name: 1577171020.doc (519K) Word count: 4419 Character count: 24548 IJISRT19DEC540 ORIGINALITY REPORT 25% 11% 9% 23% SIMILARITY INDEX INTERNET SOURCES PUBLICATIONS STUDENT PAPERS PRIMARY SOURCES econjournals.com 1 Internet Source 3% Submitted to Politeknik Negeri Sriwijaya 2 Student Paper 3% "The Relationship Among Superleader, 3 % Perceived Organizational Support and Work 2 Performance Mediated By Work Satisfaction and Employee", International Journal of Recent Technology and Engineering, 2019 Publication Submitted to Universitas Warmadewa 4 Student Paper 1% Submitted to Universitas Diponegoro 5 Student Paper 1% Submitted to Sriwijaya University 6 Student Paper 1% www.ijbmi.org 7 Internet Source 1% Agus Arwani. "Utilization of SIKADU on Quality 8 of Service of Academic Information Systems", INTENSIF: Jurnal Ilmiah Penelitian dan 1% Penerapan Teknologi Sistem Informasi, 2019 Publication Submitted to Universitas Nasional 9 Student Paper 1% Sri Marti Pramudena. "The effect of quality of 10 % worklife and job satisfaction on organizational 1 commitment", The Management Journal of Binaniaga, 2019 Publication Submitted to Universitas Jenderal Soedirman 11 Student Paper 1% Submitted to Swiss German University 12 Student Paper 1% mafiadoc.com 13 Internet Source 1% repository.unika.ac.id 14 Internet Source 1% es.scribd.com 15 Internet Source 1% Submitted to Universitas krisnadwipayana 16 Student Paper 1% Mustikaningrum Hidayati, Honorata Ratnawati 17 % Dwi Putranti, Muchamad Ardiansyah. <1 "Encouragement of Women's Employee Performance Based on o Modern Working Environment and Work Discipline on The Store Cashier and Sales Promotion Girl", Media Ekonomi dan Manajemen, 2019 Publication www.macrothink.org 18 Internet Source <1% issuu.com 19 Internet Source <1% Submitted to Universitas Dian Nuswantoro 20 Student Paper <1% Submitted to Udayana University 21 Student Paper <1% P M Anwar, I Budi. -

The SIBR-Thammasat 2013 Conference Program
PROGRAM (Finalized Version) DAY 1 (June 6, 2013) DAY 2 (June 7, 2013) DAY 3 (June 8, 2013) Registration 8:30- Registration 9:00-10:35 Sessions A1, B1 9:00-10:35 Sessions A2, B2 9:00-10:35 Sessions A3, B3, J3 16:00 (Day 1-3) 1-3) (Day 16:00 10:35-10:50 Tea break 10:35-10:50 Tea break 10:35-10:50 Tea break 10:50-12:00 Keynote Speech* 10:50-12:15 Sessions C2, D2, K2 10:50-12:15 Sessions C3, D3, K3 12:00-13:25 Lunch (Grand Panorama) 12:15-13:25 Lunch (Grand Panorama) 12:15-13:25 Lunch (Grand Panorama) 13:25-15:00 Sessions E1, F1, M1 13:25-15:00 Sessions E2, F2, M2 13:25-15:00 Sessions E3, F3 15:00-15:15 Tea break 15:00-15:15 Tea break 15:00-15:15 Closing & Tea gathering 15:15-16:40 Sessions G1, H1, N1 15:15-16:40 Sessions G2, H2, N2 * “Interdisciplinary: the Evolution of Research and Public Policy Planning”, by Professor Dr. Sakon Varanyuwatana, Faculty of Economics, Thammasat University (Venue: Panorama I) Session A1: ECONOMIC, SOCIAL & CULTURAL DEVELOPMENT (venue: Panorama I) Chair: Rob Alex Burnett (Webster University Thailand) At the Nexus of Economic Independence and Social Cohesion: A Conjectural Analysis of Impact of Low Input Projects to Family Life in Zimbabwe (b13-090) Speaker: Sevias Guvuriro (University of the Free State, South Africa) Growth and Business Development: Promoting New Talent within SME Fashion Design and Adding Value to the Economy in Bangladesh: Characteristics, Opportunities and Barriers to Growth (b13-091) Speaker: Lynne Hammond (London College of Fashion) Gender, Social Class and Peer Group Influences on Teenagers’ -

Mapping Research Systems in Developing Countries
Mapping research systems in developing countries Country report: the Science and Technology system in Indonesia Project Leaders: CREST: Centre for Research on Science and Technology, University of Stellenbosch, South Africa IRD: Institute for Research on Development, France 1 Table of Contents Introduction ....................................................................................................................................... 1 1. Scientific Activities in the Colonial Period ......................................................................... 2 1.1 Developments in S&T Policy Institutions after Independence, 1949 ................................. 2 2. Universities and Human Resources .................................................................................. 6 3. Indonesia’s Main Science Institutions .............................................................................. 9 4. Indonesia’s Agriculture Research ................................................................................... 11 5. Industry and High Technology ........................................................................................ 11 5.1 Aircraft Industry ............................................................................................................ 12 5.2 Biotechnology in Indonesia ............................................................................................ 12 6. Concluding Remarks ...................................................................................................... 13 7. References.................................................................................................................... -

The Influence of Destination Decision-Making When Choosing
THE INFLUENCE OF DESTINATION DECISION-MAKING WHEN CHOOSING DESTINATIONS WHICH AFFECT SATISFACTION AND CONSUMER BEHAVIOUR OF THE UNIVERSITY STUDENT IN JAKARTA: A CASE OF BALI ISLAND By Muhammad Khazana 11303070 BACHELOR’S DEGREE In BUSINESS ADMINISTRATION HOTEL AND TOURISM MANAGEMENT CONCENTRATION FACULTY OF BUSINESS ADMINISTRATION AND HUMANITIES SWISS GERMAN UNIVERSITY The Prominence Tower Jalan Jalur Sutera Barat No. 15, Alam Sutera Tangerang, Banten 15143 Indonesia August 2017 Revision after the Thesis Defense on July 18th 2017 THE INFLUENCE OF DESTINATION DECISION-MAKING Page 2 of 104 WHEN CHOOSING DESTINATIONS WHICH AFFECT SATISFACTION AND CONSUMER BEHAVIOUR OF THE UNIVERSITY STUDENT IN JAKARTA: A CASE OF BALI ISLAND STATEMENT BY THE RESEARCHER I hereby declare that this submission is my own work and to the best of my knowledge, it contains no material previously published or written by another person, nor material which to a substantial extent has been accepted for the award of any other degree or diploma at any educational institution, except where due acknowledgement is made in the thesis. Muhammad Khazana ____________________________________________ Student Date Approved by: Roozana Ritonga, BBA, PG Dipl., M.Par ____________________________________________ Thesis Advisor Date Dr. Nila K. Hidayat SE., MM. ____________________________________________ Dean Date Muhammad Khazana THE INFLUENCE OF DESTINATION DECISION-MAKING Page 3 of 104 WHEN CHOOSING DESTINATIONS WHICH AFFECT SATISFACTION AND CONSUMER BEHAVIOUR OF THE UNIVERSITY STUDENT IN JAKARTA: A CASE OF BALI ISLAND ABSTRACT THE INFLUENCE OF DESTINATION DECISION-MAKING WHEN CHOOSING DESTINATIONS WHICH AFFECT SATISFACTION AND CONSUMER BEHAVIOUR OF THE UNIVERSITY STUDENT IN JAKARTA: A CASE OF BALI ISLAND By Muhammad Khazana Roozana Ritonga, BBA, PG Dipl., M.Par SWISS GERMAN UNIVERSITY In Indonesia, there are many places for holiday and Indonesia is well known as a cultural and natural attraction which make tourists want to visit Indonesia. -

List of English and Native Language Names
LIST OF ENGLISH AND NATIVE LANGUAGE NAMES ALBANIA ALGERIA (continued) Name in English Native language name Name in English Native language name University of Arts Universiteti i Arteve Abdelhamid Mehri University Université Abdelhamid Mehri University of New York at Universiteti i New York-ut në of Constantine 2 Constantine 2 Tirana Tiranë Abdellah Arbaoui National Ecole nationale supérieure Aldent University Universiteti Aldent School of Hydraulic d’Hydraulique Abdellah Arbaoui Aleksandër Moisiu University Universiteti Aleksandër Moisiu i Engineering of Durres Durrësit Abderahmane Mira University Université Abderrahmane Mira de Aleksandër Xhuvani University Universiteti i Elbasanit of Béjaïa Béjaïa of Elbasan Aleksandër Xhuvani Abou Elkacem Sa^adallah Université Abou Elkacem ^ ’ Agricultural University of Universiteti Bujqësor i Tiranës University of Algiers 2 Saadallah d Alger 2 Tirana Advanced School of Commerce Ecole supérieure de Commerce Epoka University Universiteti Epoka Ahmed Ben Bella University of Université Ahmed Ben Bella ’ European University in Tirana Universiteti Europian i Tiranës Oran 1 d Oran 1 “Luigj Gurakuqi” University of Universiteti i Shkodrës ‘Luigj Ahmed Ben Yahia El Centre Universitaire Ahmed Ben Shkodra Gurakuqi’ Wancharissi University Centre Yahia El Wancharissi de of Tissemsilt Tissemsilt Tirana University of Sport Universiteti i Sporteve të Tiranës Ahmed Draya University of Université Ahmed Draïa d’Adrar University of Tirana Universiteti i Tiranës Adrar University of Vlora ‘Ismail Universiteti i Vlorës ‘Ismail -
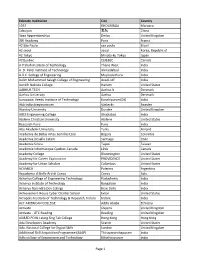
List AWS Educate Institutions
Educate Institution City Country 1337 KHOURIBGA Morocco 1daoyun 无锡 China 3aaa Apprenticeships Derby United Kingdom 3W Academy Paris France 42 São Paulo sao paulo Brazil 42 seoul seoul Korea, Republic of 42 Tokyo Minato-ku Tokyo Japan 42Quebec QUEBEC Canada A P Shah Institute of Technology Thane West India A. D. Patel Institute of Technology Ahmedabad India A.V.C. College of Engineering Mayiladuthurai India Aalim Muhammed Salegh College of Engineering Avadi-IAF India Aaniiih Nakoda College Harlem United States AARHUS TECH Aarhus N Denmark Aarhus University Aarhus Denmark Aarupadai Veedu Institute of Technology Kanchipuram(Dt) India Abb Industrigymansium Västerås Sweden Abertay University Dundee United Kingdom ABES Engineering College Ghaziabad India Abilene Christian University Abilene United States Research Pune Pune India Abo Akademi University Turku Finland Academia de Bellas Artes Semillas Ltda Bogota Colombia Academia Desafio Latam Santiago Chile Academia Sinica Taipei Taiwan Academie Informatique Quebec-Canada Lévis Canada Academy College Bloomington United States Academy for Career Exploration PROVIDENCE United States Academy for Urban Scholars Columbus United States ACAMICA Palermo Argentina Accademia di Belle Arti di Cuneo Cuneo Italy Achariya College of Engineering Technology Puducherry India Acharya Institute of Technology Bangalore India Acharya Narendra Dev College New Delhi India Achievement House Cyber Charter School Exton United States Acropolis Institute of Technology & Research, Indore Indore India ACT AMERICAN COLLEGE -

Iconent 2016 International Conference on Entrepreneurship
IConEnt 2016 International Conference on Entrepreneurship March 17th, 2016 Universitas Pelita Harapan PROCEEDINGS Organized by: Universitas Pelita Harapan (UPH) Jl. M.H. Thamrin Boulevard Lippo Village 15811 Banten Website: http://iconent.global.uph.edu/ International Conference on Entrepreneurship (IConEnt 2016) “How Innovation Could Improve the Performance and Productivity in Entrepreneurship?” Business School Universitas Pelita Harapan Published by: Office Research and Publication Business School Universitas Pelita Harapan Jl. M.H. Thamrin Boulevard Lippi Village 15811 Banten Tangerang, Indonesia Email: [email protected] ; [email protected] Telp: +6221 5460901 Ext. 1255 Website: http://iconent.global.uph.edu/ This publication is copyright. Subject to statutory exception and to the provisions of relevant collective licensing agreements, no reproduction of any part may take place without the written permission of the Business School Universitas Pelita Harapan. First published in 2016 Technical Editor : Greisye Magdalena, S.Kom Layout : Michelle Alviana Cover Design : Jessy Kusno ISBN : 978-979-96162-2-7 (printed version) 978-979-96162-3-4 (electronic version) Distributed by: Business School Universitas Pelita Harapan Building F, 1st floor Jl. M.H. Thamrin Boulevard Lippi Village 15811 Banten Tangerang, Indonesia Email: [email protected] ; [email protected] Telp: +6221 5460901 Ext. 1255 Website: http://iconent.global.uph.edu/ ii FOREWORD FROM THE RECTOR As rector of Universitas Pelita Harapan, I thereby express my deep satisfaction to have you as presenters and participants on the International Conference on Entrepreneurship 2016. I also express my most sincere gratifications for your special effort made to be together today. As host, I am deeply pleased to sincerely and warmly welcome our keynote speakers, Prof. -

Committee of the 2015 International Conference on Mathematics, Its Applications, and Mathematics Education
Home Search Collections Journals About Contact us My IOPscience Committee of The 2015 International Conference on Mathematics, its Applications, and Mathematics Education This content has been downloaded from IOPscience. Please scroll down to see the full text. 2016 J. Phys.: Conf. Ser. 693 011002 (http://iopscience.iop.org/1742-6596/693/1/011002) View the table of contents for this issue, or go to the journal homepage for more Download details: IP Address: 14.182.81.119 This content was downloaded on 30/06/2016 at 05:18 Please note that terms and conditions apply. ICMAME 2015 IOP Publishing Journal of Physics: Conference Series 693 (2016) 011002 doi:10.1088/1742-6596/693/1/011002 Committee of The 2015 International Conference on Mathematics, its Applications, and Mathematics Education Sudi Mungkasi Chair of The 2015 International Conference on Mathematics, its Applications, and Mathematics Education (ICMAME 2015), Sanata Dharma University, Mrican, Tromol Pos 29, Yogyakarta 55002, Indonesia E-mail: [email protected] Editor in Chief Dr. Herry Pribawanto Suryawan Department of Mathematics, Sanata Dharma University, Indonesia Associate Editors Dr. Hendra Gunawan Harno Department of Electrical and Computer Engineering, Curtin University Sarawak, Malaysia Dr. Hongki Julie Department of Mathematics Education, Sanata Dharma University, Indonesia Dr. Mahardhika Pratama School of Engineering and Mathematical Sciences, La Trobe University, Australia Dr. Tarsisius Sarkim Department of Physics Education, Sanata Dharma University, Indonesia Dr. Vikram Sunkara Department of Mathematics and Computer Science, Freie Universität Berlin, Germany Drs. Agah Drajat Garnadi, Grad.Dipl.Sc. Department of Mathematics, Bogor Agricultural University, Indonesia Ir. Ignatius Aris Dwiatmoko, M.Sc. Department of Mathematics, Sanata Dharma University, Indonesia Prof. -

Volume 03 Issue 1 2018
VOLUME 03 | ISSUE 01 | 2018 | ISSN2424872X APBioNet Newsletter HIGHLIGHTS 15th International Conference on Bioinformatics (InCoB2017), APBioNet AGM 2017 Travel Fellowship Awardees, Report from Travel Fellowship Recipient Launch of Data Science eXchange Initiative (DSXi) Inauguration of International Symposium on Bioinformatics (InSyB) A P B i o N e t Advancing Bioinformatics and Allied Newsletter Disciplines in the Asia-Pacific Region TABLE OF CONTENT Editor’s Note 1. Editor’s Note The year 2017 has been an eventful one for APBioNet! This year we witnessed several key new 2. APBioNet AGM 2017 projects being launched and the continued success of our our annual flagship International 3. InCoB2017: Conference on Bioinformatics (InCoB), held in 1. Conference Chairman Shenzhen for the first time, and second time in China. The objective of InCoB is to provide a Report platform for experts and budding bioinformaticians to discuss and exchange ideas 3. Travel Fellowship Awards and thoughts on the development of 4. Report from Fellowship bioinformatics in the Asia Pacific region, and this has been consistent throughout the 17 editions of Awardee the conference. This year, we were honoured to work with University Technology Sydney (UTS) and 5. Inauguration of International Tsinghua University to host the second InCoB in Symposium on Bioinformatics (InSyB) China. 6. ISBB First Meeting, Jakarta, Indonesia We also witnessed the inauguration of two new projects: Data Science eXchange Initiative (DSXi) in 7. Singapore Study Trip – Report January 2017, in collaboration with our MoU From APBioNet Interns partner Perdana University; and APBioNet’s second flagship networking vehicle, the 8. Launch of Data Science eXchange International Symposium on Bioinformatics (InSyB), held in Jakarta from 11 to 12 July 2017. -
German Business in Indonesia
German Business in Indonesia Edited by: Embassy of the Federal Republic of Germany Jakarta Jl. M.H. Thamrin No. 1, Jakarta 10310 – Indonesia Tel.: +62 (0)21 / 398 55 154, Fax: +62 (0)21 / 398 55 130 [email protected] http://www.jakarta.diplo.de Issue: April 2021 1 2 Inhaltsverzeichnis / Table of Contents Allgemeine Hinweise / General Remarks Zeichenerklärung / Explanation of Symbols Organisationen / Organizations Deutsche Botschaft / German Embassy Honorarkonsuln / Honorary Consuls EKONID – Deutsch-Indonesische Industrie- und Handelskammer GTAI – Gesellschaft für Außenwirtschaft & Standortmarketing mbH / Germany Trade and Invest GIZ – Deutsche Gesellschaft für International Zusammenarbeit DEG – Deutsche Investitions- und Entwicklungsgesellschaft SES – Senior Experten Service Poltische Stiftungen / Political Foundations Goethe-Institut e.V. Deutsches Haus Surabaya DAAD – Deutscher Akademischer Austauschdienst DSJ – Deutsche Schule Jakarta IULI – International University Liaison Indonesia SGUW - Swiss German University Westphalia Stiftung e.V. Kirchengemeinden Die Brücke – Yayasan Sosial Jembatan Elektronische Portale / Electronic Portals Deutsche Firmen von A bis Z / German Companies in Alphabetical Order Deutsche in weiteren Firmen / German Citizens in Other Companies 3 Allgemeine Hinweise / General Remarks Die Telefon- und Telefaxnummern in Indonesien und Deutschland werden jeweils für die nationale Anwahl angegeben. Die internationalen Vorwahlen sind für Indonesien +62 für Deutschland +49 Phone and fax numbers in Indonesia and Germany are indicated for national access. For international access from Indonesia to Germany please replace the initial digit „0“ with the country code for Germany (+49). Die Zeitverschiebung Jakartas zu Deutschland beträgt +6 Std während der deutschen Winterzeit +5 Std während der deutschen Sommerzeit. The German time zone is WIB -6 during German winter time (November - March) WIB -5 during German summer time (April - October) Für die Vollständigkeit und Richtigkeit der Angaben kann die Botschaft keine Gewähr übernehmen. -
Agenda and Parallel Session Schedule
EECSI 2016 PROGRAM SCHEDULE International Conference on Electrical Engineering, Computer Science and Informatics (EECSI 2016) Patra Jasa Semaranng Convention Hotel, Indonesia WEDNESDAY 23rd November 2016 Time Session 07.30 – 08.00 Registration 08.00 – 08.05 Opening 08.05 – 08.10 The reading of The Holy Al‐Qur’an 08.10 – 08.30 Welcome Speech Director of IAES Rector of UNISSULA 08.30 – 08.40 MOU letter signature 08.40 – 08.50 Photo session 08.50 – 09.00 Coffee Break Keynote Speech 09.00 – 10.00 Prof. Dr. Nadia Magnenat Thalmann Professor of Robootics & SiGnal ProcessinG, NTU Singapore and MIRALab University of Geneva, Geneva, SwitzerlanD 10.00 – 11.00 Prof. Dr. Ali Selamat Professor of Computer Science , Faculty of ComputtinG, Universiti TeknoloGi Malaysia 11.00 – 12.00 Dr. Ir. Pekik Argo Dahono Associate Professor of Power Electronics , Institute of TechnooloGy BanDunG, InDonesia 12.00 – 13.00 Lunch Parallel PPresentation Sessions 13.00 – 14.40 Parallel Session 1 14.40 – 15.10 Coffee break 15.10 – 17.00 Parallel Session 2 GALA DINNER 19.00 – 22.00 Welcomee speech from EECSI 2016 chief Conferencce awards for best paper and best presentation Conferencce dinner Closing ceremony Thursday 24th November 2016 Time Session 8:00 - 12.00 Workshop on Successful Journal Publication “How to ExtenD Conference Paper to Journal Publication” Speakers: Assoc. Prof. Dr. Tole Sutikno Assoc. Prof. Moch Facta Dr. Munawar RiyaDi 14.00-15.00 cultural program (city tours)* (*with additional arrangement) PARALEL SESSION ROOM 1 Parallel Session 1 No Time ID-Code Title Presenter 1 13:00-13:10 CAIR-153 Differential-Drive Mobile Robot Siti Nurmaini Control Design based-on Linear Robotic and Control Research Lab. -

School Profile - SMA Negeri 8 Jakarta
School Profile - SMA Negeri 8 Jakarta About SMA Negeri 8 Jakarta is 3-year a public high school in South Jakarta, Indonesia. It is located on Jalan Taman Bukit Duri, South Jakarta. The school received Accreditation ‘A’ from National Accreditation Board (BAN). Founded in August 1, 1958, SMA Negeri 8 Jakarta is well known for its highly competitive admission process, achievements in science competitions such as the National Science Olympiad, and an excellent performance of its students in the Indonesian public university entrance examination. The school has been ranked as one of the best high schools in the country for multiple times, and recently ranked 4th nationally and 2nd highest in Jakarta province based on the average score on National University Placement Test (UTBK) 2020. Many of its students study in University of Indonesia and Bandung Institute of Technology, considered Indonesia's most prestigious colleges along with Gadjah Mada University, as well as universities abroad, many of them pursue degrees in medicine, engineering and economics. Vision Creating high quality graduates in the aspects of personality, academics and non-academics of international standard Mission • Organizing learning activities that are interactive, inspirational, fun, challenging students to participate actively and creatively. • Organizing a learning system that encourages actualization of student competencies. • Carrying out personality development activities that include faith and noble morals. • Organizing interest and talent development activities based on needs and achievement- oriented. • Implement efficient, effective, transparent and accountable school governance. Curriculum SMAN 8 received Accreditation ‘A’ from the National Accreditation Body/BAN. The school is using Indonesia National Curriculum 2013 that lasts for 3 years (10th to 12th grade) in which sudents should enroll in specialization of natural sciences or social sciences from the 10th grade.