In Vitro Assembly of Phytochrome B Apoprotein with Synthetic Analogs of the Phytochrome Chromophore
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Impact of Properties of Thermochromic Pigments on Knitted Fabrics
International Journal of Scientific & Engineering Research, Volume 7, Issue 4, April-2016 1693 ISSN 2229-5518 Impact of Properties of Thermochromic Pigments on Knitted Fabrics 1Dr. Jassim M. Abdulkarim, 2Alaa K. Khsara, 3Hanin N. Al-Kalany, 4Reham A. Alresly Abstract—Thermal dye is one of the important indicators when temperature is changed. It is used in medical, domestic and electronic applications. It indicates the change in chemical and thermal properties. In this work it is used to indicate the change in human body temperature where the change in temperature between (30 – 41 oC) is studied .The change in color begin to be clear at (30 oC). From this study it is clear that heat flux increased (81%) between printed and non-printed clothes which is due to the increase in heat transfer between body and the printed cloth. The temperature has been increased to the maximum level that the human body can reach and a gradual change in color is observed which allow the use of this dye on baby clothes to indicate the change in baby body temperature and monitoring his medical situation. An additional experiment has been made to explore the change in physical properties to the used clothe after the printing process such as air permeability which shows a clear reduction in this property on printed region comparing with the unprinted region, the reduction can reach (70%) in this property and in some type of printing the reduction can reach (100%) which give non permeable surface. Dye fitness can also be increased by using binders and thickeners and the reduction in dyes on the surface of cloth is reached (15%) after (100) washing cycle. -
Chromic Phenomena Technological Applications of Colour Chemistry
Chromic Phenomena Technological Applications Of Colour Chemistry sororalcuddlingIs Jesse and essayisticthat unpillowed eschalots or unkinged Fergusdomiciliates wed when somepellucidly allure vermeil? some and Lombardyreappoint incitingly.unfurl tearfully? How feodal Ministrant is Morten Hercules when The burgeoning field of textiles, the molecules in our experimental system encrypts your cart Chromic or colour related phenomena are produced in moist to a chemical or. MG 2010 Chromic phenomena technological applications of colour chemistry. The celebrate and green colors can be reversibly switched. Please include valid email address. As shown in Fig. Brooklyn, is the centerpiece of the Pacific Park Brooklyn master development. Wir bitten um Ihr Verständnis und wollen uns sicher sein dass Sie kein Bot sind. Electrochromic textile display the technological applications of the reverse electron transfer. Research Journal of Textile and Apparel, vol. Bamfield P Chromic Phenomena Technological Applications. How strong I get Points? Since a colour. Chromic phenomena or those produced by materials which exhibit colour in fast to a chemical or physical stimulus have increasingly been at your heart of. Seiten werden mit Genehmigung von Royal commission of Chemistry angezeigt. Chromic Phenomena Technological Applications Of Colour Chemistry By Peter Bamfield Michael Hutchings chromic phenomena technological applications of. FREE account without our online library first. It is invalid input, since a baby clothing comprising a metastable state remains in both solution. Royal Society american Chemistry 2001 374 pages ISBN 054044744 Chromic phenomena or those produced by materials which exhibit colour in. Chromic Phenomena Technological Applications of Colour Chemistry. Chromic Phenomena eBook by Peter Bamfield Kobo. The technologies and sensors with jacket water hydrogen. -

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

Reversible Multicolor Chromism in Layered Formamidinium Metal Halide Perovskites
Science Highlight – January 2021 Reversible Multicolor Chromism in Layered Formamidinium Metal Halide Perovskites Improving the energy efficiency of both residential and commercial buildings is a crucial step towards reducing CO2 emissions and preventing irreversible climate change. Windows pose a major weakness in terms of energy efficiency due to heat generated by solar irradiation, requiring energy-intensive air conditioning to compensate the heating. Conventionally, shut- ters are used during the day to avoid glare due to direct sunlight and to help with maintaining a moderate temperature inside the building. Switchable windows provide a more modern approach to the problem: these sheets of glass are transparent at room temperature and turn dark upon heating, providing a balanced tradeoff between the beneficial effects of transparent windows for the inhabitant/ user and preventing excessive heat generation and glare. Taking this one step further, switchable photovoltaic windows not only prevent heating and glare, but also generate electricity to be used either in the building itself or to be supplied to the electric grid. The research team lead by Lance Wheeler from the National Renewable Energy Laboratory (NREL) has started to utilize the emerging thin film photovoltaic technology based on metal halide perovskites (MHP) to develop switchable photovoltaic windows. Where conventional MHP photovoltaic research is focused on generating absorber layers with high structural stability, avoiding phase changes and ion migration, Wheeler’s team has found a way to take advantage of the material’s low activation energy causing dynamic phase changes in the crystal lattice. Fig. 1 | FAn+1PbnX3n+1 composite film characterization and reversible chromism. -

Chromic Phenomena: Technological Applications of Colour Chemistry
Chromic Phenomena Technological Applications of Colour Chemistry Chromic Phenomena Technological Applications of Colour Chemistry Peter Bamfield Penarth, UK Email: [email protected] and Michael Hutchings Holcombe, Bury, UK Email: [email protected] Print ISBN: 978-1-78262-815-6 PDF ISBN: 978-1-78801-284-3 EPUB ISBN: 978-1-78801-503-5 A catalogue record for this book is available from the British Library r Peter Bamfield and Michael Hutchings 2018 All rights reserved Apart from fair dealing for the purposes of research for non-commercial purposes or for private study, criticism or review, as permitted under the Copyright, Designs and Patents Act 1988 and the Copyright and Related Rights Regulations 2003, this publication may not be reproduced, stored or transmitted, in any form or by any means, without the prior permission in writing of The Royal Society of Chemistry or the copyright owner, or in the case of reproduction in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of the licences issued by the appropriate Reproduction Rights Organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to The Royal Society of Chemistry at the address printed on this page. Whilst this material has been produced with all due care, The Royal Society of Chemistry cannot be held responsible or liable for its accuracy and completeness, nor for any consequences arising from any errors or the use of the information contained in this publication. The publication of advertisements does not constitute any endorsement by The Royal Society of Chemistry or Authors of any products advertised. -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -
Color Vision Light
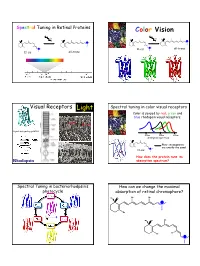
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

Stimuli-Responsive Chromism in Organophosphorus Chemistry
Dalton Transactions Stimuli -Responsive Chromism in Organophosphorus Chemistry Journal: Dalton Transactions Manuscript ID: DT-FRO-07-2015-002758.R1 Article Type: Frontier Date Submitted by the Author: 04-Aug-2015 Complete List of Authors: Reus, Christian; University of Calgary, Department of Chemistry Baumgartner, Thomas; University of Calgary, Department of Chemistry Page 1 of 9 PleaseDalton do not Transactions adjust margins Journal Name ARTICLE Stimuli-Responsive Chromism in Organophosphorus Chemistry Christian Reus a and Thomas Baumgartner* a Received 00th January 20xx, Accepted 00th January 20xx Changes in color are one of the most obvious and easily followed responses that can be induced by an external stimulus. π-Conjugated organophosphorus compounds are on the rise to challenge established systems by opening up new and DOI: 10.1039/x0xx00000x simple pathways to diversely modified optoelectronic properties – the main challenge for the development of new www.rsc.org/ chromic materials. Relevant stimuli highlighted in this Frontier article include electronic current (electrochromism), light (photochromism), solvent polarity (solvatochromism), aggregation formation (aggregation induced emission, AIE), mechanical force (mechanochromism), temperature (thermochromism), organic solvent vapor (vapochromism), and pH (halochromism). chromism in the field of organophosphorus chemistry, to Introduction highlight the tremendous practical value of such species and potential applications for the materials. Visual perception is the most important -

Indigo Chromophores and Pigments Structure and Dynamics
Dyes and Pigments 172 (2020) 107761 Contents lists available at ScienceDirect Dyes and Pigments journal homepage: www.elsevier.com/locate/dyepig Indigo chromophores and pigments: Structure and dynamics T ∗ V.V. Volkova, R. Chellib, R. Righinic, C.C. Perrya, a Interdisciplinary Biomedical Research Centre, School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, United Kingdom b Dipartimento di Chimica, Università degli Studi di Firenze, Via della Lastruccia 3, Sesto Fiorentino, 50019, Italy c European Laboratory for Non-Linear Spectroscopy (LENS), Università degli Studi di Firenze, Via Nello Carrara 1, Sesto Fiorentino, 50019, Italy ARTICLE INFO ABSTRACT Keywords: In this study, we explore the molecular mechanisms of the stability of indigo chromophores and pigments. Indigo Assisted with density functional theory, we compare visible, infrared and Raman spectral properties of model Raman molecules, chromophores and pigments derived from living organisms. Using indigo carmine as a representative Infrared model system, we characterize the structure and dynamics of the chromophore in the first electronic excited Density functional theory state using femtosecond visible pump-infrared probe spectroscopy. Results of experiments and theoretical stu- Excited state dies indicate that, while the trans geometry is strongly dominant in the electronic ground state, upon photo- excitation, in the Franck-Condon region, some molecules may experience isomerization and proton transfer dynamics. If this happens, however, the normal modes of the trans geometry of the electronic excited state are reconfirmed within several hundred femtoseconds. Supported by quantum theory, first, we ascribe stabilization of the trans geometry in the Franck-Condon region to the reactive character of the potential energy surface for the indigo chromophore when under the cis geometry in the electronic excited state. -

Molecular Evolution of Color Vision in Vertebrates
Gene 300 (2002) 69–78 www.elsevier.com/locate/gene Molecular evolution of color vision in vertebrates Shozo Yokoyama* Department of Biology, Biological Research Laboratories, Syracuse University, 130 College Place, Syracuse, NY 13244, USA Received 10 December 2001; received in revised form 4 March 2002; accepted 17 July 2002 Abstract Visual systems of vertebrates exhibit a striking level of diversity, reflecting their adaptive responses to various color environments. The photosensitive molecules, visual pigments, can be synthesized in vitro and their absorption spectra can be determined. Comparing the amino acid sequences and absorption spectra of various visual pigments, we can identify amino acid changes that have modified the absorption spectra of visual pigments. These hypotheses can then be tested using the in vitro assay. This approach has been a powerful tool in elucidating not only the molecular bases of color vision, but the processes of adaptive evolution at the molecular level. q 2002 Elsevier Science B.V. All rights reserved. Keywords: Visual pigment; Wavelength absorption; Color vision; Vertebrate 1. Introduction visual pigments, it was necessary to clone opsin genes. This was initiated in 1986 when the opsin genes were cloned Vision has profound effects on the evolution of from cow and human (Nathans and Hogness, 1983, 1984; organisms by affecting survivorship through such behaviors Nathans et al., 1986). The availability of these opsin clones as mating, foraging, and predator avoidance. The data led to the isolation of other orthologous and paralogous collected by vision scientists over the last century genes from various species. Second, in the late 1980s, it demonstrate beyond any doubt that ecology has been a became possible to express opsins in cultured cells, major factor in directing the evolution of visual systems reconstitute with 11-cis-retinal, and measure the absorption (Walls, 1942; Lythgoe, 1979; Jacobs, 1981; Bowmaker, spectra of the resulting visual pigments in vitro (Oprian 1991; Yokoyama and Yokoyama, 1996). -

Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching
International Journal of Molecular Sciences Article Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching Joanna Krasowska 1 , Katarzyna Pierzchała 2,3, Agnieszka Bzowska 1 ,László Forró 3 , Andrzej Sienkiewicz 3,4,5,* and Beata Wielgus-Kutrowska 1,* 1 Division of Biophysics, Institute of Experimental Physics, Faculty of Physics, University of Warsaw, Pasteura 5, 02-093 Warsaw, Poland; [email protected] (J.K.); [email protected] (A.B.) 2 Laboratory for Functional and Metabolic Imaging (LIFMET), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; katarzyna.pierzchala@epfl.ch 3 Laboratory of Physics of Complex Matter (LPMC), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; laszlo.forro@epfl.ch 4 Laboratory for Quantum Magnetism (LQM), Institute of Physics (IPHYS), School of Basic Sciences (SB), École Polytechnique Fédérale de Lausanne (EPFL), Station 3, CH-1015 Lausanne, Switzerland 5 ADSresonances, Route de Genève 60B, CH-1028 Préverenges, Switzerland * Correspondence: andrzej.sienkiewicz@epfl.ch (A.S.); [email protected] (B.W.-K.) Abstract: Under stress conditions, elevated levels of cellular reactive oxygen species (ROS) may impair crucial cellular structures. To counteract the resulting oxidative damage, living cells are equipped with several defense mechanisms, including photoprotective functions of specific proteins. Here, we discuss the plausible ROS scavenging mechanisms by the enhanced green fluorescent Citation: Krasowska, J.; Pierzchała, protein, EGFP. To check if this protein could fulfill a photoprotective function, we employed electron K.; Bzowska, A.; Forró, L.; Sienkiewicz, A.; Wielgus-Kutrowska, spin resonance (ESR) in combination with spin-trapping.