Habitat Structures Rainbow Trout Population Dynamics Across Spatial Scales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Bibliography of Scientific Information on Fraser River Basin Environmental Quality
--- . ENVIRONMENT CANADA — b- A BIBLIOGRAPHY OF SCIENTIFIC INFORMATION ON FRASER RIVER BASIN ENVIRONMENTAL QUALITY . 1994 Supplement e Prepared on contract by: Heidi Missler . 3870 West 11th Avenue Vancouver, B.C. V6R 2K9 k ENVIRONMENTAL CONSERVATION BRANCH PACIFIC AND YUKON REGION NORTH VANCOUVER, B.C. L- ,- June 1994 DOE FRAP 1994-11 *- \- i — --- ABSTRACT -. -. This bibliography is the third in a series of continuing reference books on the Fraser River watershed. It includes 920 references of scientific information on the environmental I quality of the Fraser River basin and is both an update and an extension of the preceding -. bibliography printed in 1992. ,= 1- ,- . 1- 1- !- 1 - — ii — RESUME — La presente bibliographic est la troiseme clans une serie continue portant sur le bassin du fleuve Fraser. Elle comprend 920 citations scientifiques traitant de la qualite de l’environnement clans le bassin du fleuve Fraser, et elle constitue une mise a jour de la bibliographic precedence, publiee en 1992. — — — ---- — —. .— — — ,- .— ... 111 L TABLE OF CONTENTS Page Abstract ‘ i Resume ii Introduction iv References Cited v Acknowledgements vi Figure: 1. Fraser River Watershed Divisions , vii ... Tables: 1. Reference Locations Vlll 2. Geographic Location Keywords ix 3. Physical Environment Keywords x 4. Contamination Kefiords xi, 5. Water Quality Keywords xii . ... 6. Natural Resources Keywords Xlll 7. Biota Keywords xiv 8. General Keywords xv Section One: Author Index Section Two: Title Index \ 117 ( L iv INTRODUCTION This bibliography is the third in a series of continuing reference books on the Fraser River watershed. With its 920 references of scientific information on the environmental quality of the , -. -

Lode-Goijd Deposits
BRITISH COLUMBIA DEPARTMENT OF MINES HON. E. C. CARSON, Minisfer JOHN F. WALKER, Deputy Minister . BULLETIN NO. 20-PART 11. LODE-GOIJD DEPOSITS South-eastern British Columbia by W.H. MATHEWS PREFACE. Bulletin 20, designed for the use of thoseinterested in the discovery of gold- bearing lode deposits, is being published as a series of separate parts. Part I. is to contain information about lode-gold production in British Columbia as awhole, and will be accompanied by a map on which the generalized geology of the Province is rep- resented. The approximate total production of each lode-gold mining centre, exclusive of by-product gold, is also indicated on the map. Each of the other parts deals with a , major subdivision of the Province, giving information about the geology, gold-bearing lode deposits, and lode-gold production of areas within the particular subdivision. In all, seven parts are proposed:- PARTI.-General re Lode-gold Production in British Columbia. PART11.-South-eastern Britis:h Columbia. ’ PART111.-Central Southern British Columbia. PART1V.-South-western British Columbia, exclusive of Vancouver Island, PARTV.-Vancouver Island. PARTVI.-North-eastern British Columbia, including the Cariboo and Hobson Creek Areas. PARTVI1.-North-western British Columbia. Bykind permission of Professor H. C. Gunning,Department ofGeology, Uni- versity of British Columbia, his compilation of the geology of British Columbia has been follo-wed in the generalized geology represented on the map accompanying Part I. Professor Gunning’s map was published in “The Miner,” Vancouver, B.C., June-July, 1943, and in “The Northern Miner,” Toronto, Ont., December 16th, 1943. -

Evaluation of Techniques for Flood Quantile Estimation in Canada
Evaluation of Techniques for Flood Quantile Estimation in Canada by Shabnam Mostofi Zadeh A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Doctor of Philosophy in Civil Engineering Waterloo, Ontario, Canada, 2019 ©Shabnam Mostofi Zadeh 2019 Examining Committee Membership The following are the members who served on the Examining Committee for this thesis. The decision of the Examining Committee is by majority vote. External Examiner Veronica Webster Associate Professor Supervisor Donald H. Burn Professor Internal Member William K. Annable Associate Professor Internal Member Liping Fu Professor Internal-External Member Kumaraswamy Ponnambalam Professor ii Author’s Declaration This thesis consists of material all of which I authored or co-authored: see Statement of Contributions included in the thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. iii Statement of Contributions Chapter 2 was produced by Shabnam Mostofi Zadeh in collaboration with Donald Burn. Shabnam Mostofi Zadeh conceived of the presented idea, developed the models, carried out the experiments, and performed the computations under the supervision of Donald Burn. Donald Burn contributed to the interpretation of the results and provided input on the written manuscript. Chapter 3 was completed in collaboration with Martin Durocher, Postdoctoral Fellow of the Department of Civil and Environmental Engineering, University of Waterloo, Donald Burn of the Department of Civil and Environmental Engineering, University of Waterloo, and Fahim Ashkar, of University of Moncton. The original ideas in this work were jointly conceived by the group. -

2010 Nesting Season British Columbia Nest Record Scheme
BRITISH COLUMBIA NEST RECORD SCHEME 56th Annual Report 2010 Nesting Season British Columbia Nest Record Scheme 56th Annual Report - 2010 Nesting Season compiled by R. Wayne Campbell, Linda M. Van Damme, Mark Nyhof, Patricia Huet Biodiversity Centre for Wildlife Studies Report No. 13 May 2011 Contents Biodiversity and Breeding Birds..............................................................................1 The 2010 Nesting Season..........................................................................................4 Summary...........................................................................................................4 Noteworthy Events...........................................................................................6 New Breeding Species..............................................................................6 Range Expansion and Isolated Nesting......................................................7 Early and Late Nesting Dates....................................................................9 Nesting Failures.....................................................................................12 Unusual Nest Sites.................................................................................15 Noteworthy Species Information Since The Birds of British Columbia..........18 Highlights.........................................................................................................23 Families and Species..............................................................................23 Brown-headed -

Wells Gray Park Master Plan
2-2-4-1-27 WELLS GRAY PARK MASTER PLAN February, 1986 Ministry of Lands Parks & Housing Parks & Outdoor Recreation Div. i TABLE OF CONTENTS PLAN HIGHLIGHTS PLAN ORGANIZATION SECTION 1 - PARK ROLE 1 1.1 INTRODUCTION 1 1.2 THE ROLE OF WELLS GRAY PARK 5 1.2.1 Regional and Provincial Context 5 1.2.2 Conservation Role 5 1.2.3 Recreation Role 7 1.3 ZONING 8 SECTION 2 - PARK MANAGEMENT 12 2.1 NATURAL RESOURCE MANAGEMENT OBJECTIVES AND POLICIES 12 2.1.1 Land and Tenures (a) Park Boundaries 12 (b) Inholdings and Other Tenures 14 (c) Trespasses 14 2.1.2 Water (a) General Principle 16 (b) Impoundment, Diversion, etc. 16 2.1.3 Vegetation (a) General Principle 16 (b) Current Specific Policies 16 2.1.4 Wildlife (a) General Principle 18 (b) Current Specific Policies 19 2.1.5 Fish (a) General Principle 21 (b) Current Specific Policies 21 2.1.6 Cultural Heritage (a) General Principle 22 (b) Current Specific Policies 22 2.1.7 Visual Resources (a) General Principle 23 (b) Current Specific Policies 23 2.1.8 Minerals Resources (a) General Principle 24 ii 2.2 VISITOR SERVICES OBJECTIVES AND POLICIES 24 2.2.1 Introduction (a) General Concept 24 (b) Access Strategy 26 (c) Information & Interpretation Strategy 26 2.2.2 Visitor Opportunities 26 (a) Auto-access Sightseeing and Touring 26 (b) Auto-access Destination 28 (c) Visitor Information Programs 28 (d) Winter Recreation 31 (e) Wild River Recreation 31 (f) Motorboat Touring 32 (g) Angling 32 (h) Hunting 32 (i) Hiking 33 (j) Canoeing 33 (k) Horseback Riding 34 (1) Alpine Appreciation 34 (m) Research 34 2.2.3 -
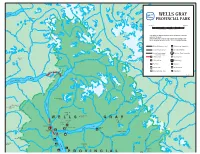
Download a PDF of the Map Below
r C r n C i t r a M a iw K k c a l L B Mitchell Christian C r L MT e t WINDER e r T C kilometres kilometres R a 5 0 2 4 6 8 10 u s h ELEVATIONS IN METRES ABOVE SEA LEVEL Pe nfo ld C The intent of this brochure is to be used as a reference r MT GOODALL guide to the park. MT A more detailed contour map can be purchased at the BEAMAN Wells Gray Information Centre. Phone: (250) 674-2646 R Major Highway, paved Wilderness Campsites a r a g Local Road, paved a Group Camping i N r C Local Road, gravel Vehicle / Tent Campsites (4WD where indicated) Hiking Trail Sani Station R NIAGARA Cr g PK on in obs Hiking Route Picknicking ar d H o ea R L E lla Parking Shelter F r e ye u l Horse Trail Boat Launch B C r Cr Summit Mountain Bike Trail Viewpoint C mit r um Lake L S it m T m r E u a ast S i Revised 2001 x l Lyn Cr Stranger L L A K E TWIN N E L 728 m S E SPIRES U B Q ra N or r it th B h R C e w i k l a l h a i t p L e l o d A M 858 m in Cr e r T hom C ps r on MT HUGH NEAVE r 2829 m C r C K R nutso n r n L C ic o k s s O k b ISOSCELES MTN i v l o 2430 m le i s t DUTCHMAN MTN H oat 2376 m G e r u r EAGLES NEST z C u A a e PK t GARNET PK n C a r 2860 m M ne BUCHANAN PK or C 2469 m H r W E L L S G R A Y MT HOGUE BATOCHE s MT HUNTLEY 2396 m u PK g n 2429 m A Cr Sundt Falls Lake MT HUTCH Huntley Col Route e Rainbow 2519 m B ur re ar z Falls ie el pr la Hobson Lake A m C r Le wkley Trail 15 km Ha Angus Horne 682 m An g Portage u L s R M 990 m a c r K C H C a r orne y MT PERSEUS e tl r u M F l e EUREKA PK C u b l a 2428 m e AZURE MTN i a x R V r w 2495 m -

Wells Gray Park
reconnaissance and preliminary recreation plan wells gray park by c.p. lyons Parks Section forest economics division b.c. Forest Service – 1941 – Reference No. p41 General file: 0135867 preface Wells Gray Park is an outstanding potential recreational area; it has remarkable scenic attractions as well as exceptional fishing, big game hunting and wilderness area possibilities. The Park is located about 100 miles north of Kamloops in the Kamloops Forest District and is accessible by the Caribou and North Thompson River regions. The proper development of this area as a Provincial Park depends upon planned recreational management in order to adequately provide for present and future use. With this objective in mind, a reconnaissance of the Park was made in 1940 by Mr. C.P. Lyons, whose report and preliminary recreation plan is herein detailed. Sufficient information is now available to introduce planned management but it is still necessary to make further investigations before extensive developments take place. For the time being commercial lodge and campsite privileges on Crown land should be restricted to a minimum and, if possible, confined to guides of hunting and fishing parties who now make use of the Park. It would be particularly advisable to limit locations for commercial enterprises to the specific regions detailed in this report and to stipulate a maximum value for construction and development work. By so doing adjustments could be more easily made to suit any detailed management plan which may be prepared in the future. Existing private use on Crown land should be brought under a permit system and there is no reason why expansion in this direction should not be encouraged. -

EVOLUTION of the NORTH AMERICAN CORDILLERA William R. Dickinson
7 Apr 2004 20:19 AR AR211-EA32-02.tex AR211-EA32-02.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.earth.32.101802.120257 Annu. Rev. Earth Planet. Sci. 2004. 32:13–45 doi: 10.1146/annurev.earth.32.101802.120257 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on November 10, 2003 EVOLUTION OF THE NORTH AMERICAN CORDILLERA William R. Dickinson Department of Geosciences, University of Arizona, Tucson, Arizona 85721; email: [email protected] Key Words continental margin, crustal genesis, geologic history, orogen, tectonics ■ Abstract The Cordilleran orogen of western North America is a segment of the Circum-Pacific orogenic belt where subduction of oceanic lithosphere has been under- way along a great circle of the globe since breakup of the supercontinent Pangea began in Triassic time. Early stages of Cordilleran evolution involved Neoproterozoic rifting of the supercontinent Rodinia to trigger miogeoclinal sedimentation along a passive continental margin until Late Devonian time, and overthrusting of oceanic allochthons across the miogeoclinal belt from Late Devonian to Early Triassic time. Subsequent evolution of the Cordilleran arc-trench system was punctuated by tectonic accretion of intraoceanic island arcs that further expanded the Cordilleran continental margin during mid-Mesozoic time, and later produced a Cretaceous batholith belt along the Cordilleran trend. Cenozoic interaction with intra-Pacific seafloor spreading systems fostered transform faulting along the Cordilleran continental margin and promoted incipient rupture of continental crust within the adjacent continental block. INTRODUCTION Geologic analysis of the Cordilleran orogen, forming the western mountain system of North America, raises the following questions: 1. -

Reclaiming Our Spirits: Community Theatre on February 21St Tory Resolution of the Resi- the Three Parties Agreed to Dential School Issue
,j a I .., ,c,or. ,.iÿb.ü-ó *' L. lark , Le e a .v 1 r --,. VOA .44 f 1':-1 r- MIR hi Canadian Publications Mail Product Shilth -Sa Sa Ha- Agreement No. 467510 VOL.23 NO. 2, FEBRUARY 22.1996 Nuu- chah -nulth for "Interesting News" Sales e s ruary = o try o 11.1 Prior to a Party exer- come to an agreement on cising its right to suspend Negotiators Initial including residential negotiations under section Nuu- chah -nulth schools as an issue. Nuu - 12.1, the Parties shall in Framework chah -nulth negotiators good faith make all rea- how sonable efforts to enter into Agreement continued to stress important this issue is to appropriate methods of After two and a their people, especially to dispute resolution. half days of hard bargain- those who are still suffer- As all outstand- ing the Nuu- chah -nulth ing from abuses while at- ing issues were addressed, Tribal Council Treaty tending the residential the Nuu- chah -nulth Co- F Table came to an agree- schools. Chief Negotiators and the ment on the residential The Federal Chief Negotiators for school issue that First Na- Government's Chief Nego- Canada and British Colum- tions insisted on having in tiator Wendy Porteous bia initialled the February the framework agreement. said at the opening session 21st version of the Frame- All of the other that she did not have the work Agreement. The ini- substantive issues in the mandate to negotiate com- tialled Framework Agree- framework agreement pensation, an apology or ment is being recom- were agreed to by the Nuu - the redress of historical mended for approval to chah-nulth, the Federal and wrongs relating to residen- those authorized to sign on ._ the Provincial govern- tial schools. -

U.S. Geological Survey Bulletin 1056-B
Index to the Geologic Names of North America By DRUID WILSON, GRACE C. KEROHER, and BLANCHE E. HANSEN GEOLOGIC NAMES OF NORTH AMERICA GEOLOGICAL SURVEY BULLETIN 10S6-B Geologic names arranged by age and by area containing type locality. Includes names in Greenland, the West Indies, the Pacific Island possessions of the United States, and the Trust Territory of the Pacific Islands UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1959 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.G. - Price 60 cents (paper cover) CONTENTS Page Major stratigraphic and time divisions in use by the U.S. Geological Survey._ iv Introduction______________________________________ 407 Acknowledgments. _--__ _______ _________________________________ 410 Bibliography________________________________________________ 410 Symbols___________________________________ 413 Geologic time and time-stratigraphic (time-rock) units________________ 415 Time terms of nongeographic origin_______________________-______ 415 Cenozoic_________________________________________________ 415 Pleistocene (glacial)______________________________________ 415 Cenozoic (marine)_______________________________________ 418 Eastern North America_______________________________ 418 Western North America__-__-_____----------__-----____ 419 Cenozoic (continental)___________________________________ 421 Mesozoic________________________________________________ -

2008 Population Census of Mountain Caribou in Wells Gray Park, The
2008 Population Census of Mountain Caribou in Wells Gray Park, the North Thompson Watershed and a portion of the Adams River Watershed of the Ministry of Environment Thompson Region Kelsey L. Furk March 2008 Prepared for: BC Ministry of Environment, Thompson Region and BC Ministry of Forests Research Branch 1 Executive Summary Staff and Contractors from the Ministry of Environment, Thompson Region conducted a total count census of part of the Wells Gray and Allan subpopulations and all of the Groundhog subpopulation on March 6 th , 7 th , 31st and April 1st and 2nd. Coverage of the survey area was complete, however, survey conditions were variable overall, with good tracking and weather conditions for some areas (the south part of Wells Gray Park), and poor tracking (Miledge, Allan and MU 3-40-Berry Creek) or weather conditions (north part of Wells Gray Park) in some areas. The coverage area was similar to 2006, but more hours were spent searching, due to poor weather, and difficult tracking conditions. Mountain Caribou in the Headwaters Forest District were studied using radio-telemetry from 1995 to present. The small number of radio-collared caribou remaining in the area (n=8) precluded making population estimates with confidence intervals for this year. Surveys conducted prior to 2002 reflected jurisdictional rather than ecological boundaries. However, where possible, the 2008 survey results are compared to previous surveys (summarized in Furk (2006)). There were fewer caribou counted overall in this survey (182) than the 2006 (225) survey. However, this primarily reflects a decline in the number of caribou counted in MU-3-44 south of the North Thompson River (Miledge Creek). -

Minister of Mines and Petroleum Resources PROVINCE of BRITISH COLUMBIA
Minister of Mines and Petroleum Resources PROVINCE OF BRITISH COLUMBIA ANNUAL REPORT for the Year Ended December 3 1 1963 BRITISH COLUMBIA DEPARTMENT OF MINES AND PETROLEUM RESOURCES VICTORIA, B.C. HON. DONALD L. BROTHERS, Minister. P. .I. MULCAHY, Deputy Minister. J. W. PECK, Chief Inspector of Mines. S. METCALFE, Chief Analyst and Assayer. HARTLEY SARGENT, Chief. Mineralogical Branch. K. B. BLAKEY, Chief Gold Commissioner and Chief Commissioner, Petroleum and Natural Gas. J. D. LINEHAM, Chief, Petroleum and Natural Gas Conservation Branch. Major-General the Honourable GEORGE RANWLPH PEARICES, V.C., P.C., C.B., D.S.O., M.C., Lieutenant-Governor of the Province of British Columbia. MAY IT PLEASE YOUR HONOUR: The Annual Report of the Mineral Industry of the Province for the year 1963 is herewith respectfully submitted. DONALD L. BROTHERS, Minister of Mines and Petroleum Resources. Minister of Mines and Petroleum Resources Ofice, March 31, 1964. Robert Blair King, Inspector and Resident Engineer from 1948 to 1959, died in Vancouver on August 1, 1963. He was born in Rouleau, Sask., July 17, 1912. His early education was in his native Province, after which he graduated in mining engineering from Queen’s University in 1935. Before joining the Department in 1948 he had a varied career in mining-as an engineer at Hollinger Consolidated Gold Mines Ltd., as an instructor at the Haileybury School of Mines, and as a tie man- ager at Bayonne Consolidated Mines Ltd. He left the Department in 1959 to accept the position of Safety Director with the Mining Associa- tion of British Columbia, and in these duties established close co-opera- tion with the Department.