Experiment "Gamma Dosimetry and Dose Rate Determination" Instruction for Experiment "Gamma Dosimetry and Dose Rate Determination"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Utilization of MOSFET Dosimeters for Clinical Measurements in Radiology
The Utilization of MOSFET Dosimeters for Clinical Measurements in Radiology David Hintenlang, Ph.D., DABR, FACMP Medical Physics Program Director J. Crayton Pruitt Family Department of Biomedical Engineering J. Crayton Pruitt Family Department of Biomedical Engineering Medical Physics Program Conflict of interest statement: The presenter holds no financial interest in, and has no affiliation or research support from any manufacturer or distributor of MOSFET Dosimetry systems. J. Crayton Pruitt Family Department of Biomedical Engineering Medical Physics Program The MOSFET Dosimeter • Metal oxide silicon field effect transistor • Uniquely packaged to serve as a radiation detector – developed as early as 1974 • Applications – Radiation Therapy Dose Verification – Cosmic dose monitoring on satellites – Radiology • ~ 1998 - present J. Crayton Pruitt Family Department of Biomedical Engineering Medical Physics Program Attractive features • Purely electronic dosimeter • Provides immediate dose feedback • Integrates over short periods of time • Small size and portability • Simultaneous measurements (up to 20) J. Crayton Pruitt Family Department of Biomedical Engineering Medical Physics Program Demonstrated radiology applications – Patient dose monitoring/evaluation • Radiography • Fluoroscopic and interventional procedures • CT • Mammography J. Crayton Pruitt Family Department of Biomedical Engineering Medical Physics Program Principles of operation • Ionizing radiation results in the creation of electron-hole pairs • Holes migrate and build up -

Development of Chemical Dosimeters Development Of
SUDANSUDAN ACADEMYAGADEMY OFOF SCIENCES(SAS)SGIENGES(SAS) ATOMICATOMIC ENERGYEhTERGYRESEARCHESRESEARCHES COORDINATIONCOORDII\rATI ON COUNCILCOUNCIL - Development of Chemical Dosimeters A dissertation Submitted in a partial Fulfillment of the Requirement forfbr Diploma Degree in Nuclear Science (Chemistry) By FareedFadl Alla MersaniMergani SupervisorDr K.S.Adam MurchMarch 2006 J - - - CONTENTS Subject Page -I - DedicationDedication........ ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... I Acknowledgement ... '" ... ... ... ... ... ... '" ... ... ... ... '" ... '" ....... .. 11II Abstract ... ... ... '" ... ... ... '" ... ... ... ... -..... ... ... ... ... ... ..... III -I Ch-lch-1 DosimetryDosimefry - 1-1t-l IntroductionLntroduction . 1I - 1-2t-2 Principle of Dosimetry '" '" . 2 1-3l-3 DosimetryDosimefiySystems . 3J 1-3-1l-3-l primary standard dosimeters '" . 4 - 1-3-2l-3-Z Reference standard dosimeters ... .. " . 4 1-3-3L-3-3 Transfer standard dosimeters ... ... '" . 4 1-3-4t-3-4 Routine dosimeters . 5 1-4I-4 Measurement of absorbed dose . 6 1-5l-5 Calibration of DosimetryDosimetrvsystemsvstem '" . 6 1-6l-6 Transit dose effects . 8 Ch-2ch-2 Requirements of chemical dosimeters 2-12-l Introduction ... ... ... .............................................. 111l 2-2 Developing of chemical dosimeters ... ... .. ....... ... .. ..... 12t2 2-3 Classification of Dosimetry methods.methods .......................... 14l4 2-4 RequirementsRequiremsnts of ideal chemical dosimeters ,. ... 15 2-5 Types of chemical system . -
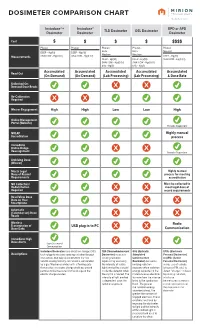
Dosimeter Comparison Chart
DOSIMETER COMPARISON CHART Instadose®+ Instadose® EPD or APD TLD Dosimeter OSL Dosimeter Dosimeter Dosimeter Dosimeter Cost $ $ $ $$$$ Photon Photon Photon Photon Photon Beta Beta Neutron DEEP - Hp(10) DEEP - Hp(10) Neutron Neutron Measurements SHALLOW - Hp(0.07) SHALLOW - Hp(0.07) DEEP - Hp(10) DEEP - Hp(10) DEEP - Hp(10) SHALLOW - Hp(0.07) SHALLOW - Hp(0.07) SHALLOW - Hp(0.07) EYE - Hp(3) EYE - Hp(3) Read Out Accumulated Accumulated Accumulated Accumulated Accumulated (On-Demand) (On-Demand) (Lab Processing) (Lab Processing) & Dose Rate Unlimited On- Demand Dose Reads Re-Calibration Required Wearer Engagement High High Low Low High Online Management Portal (Website) Provider Dependent NVLAP Highly manual Accreditation process Immediate Online Badge Reassignment Provider Dependent Archiving Dose (Wearer) Meets Legal Highly manual Dose of Record process for meeting Requirements accreditation NO Collection/ Must be collected to Redistribution meet legal dose of Required record requirements Read/View Dose Data on Your Smartphone Automatic (Calendar-set) Dose Reads Wireless Radio Transmission of USB plug-in to PC Dose Data Communication Immediate High Dose Alerts Upon Successful Communication Instadose Dosimeters use direct ion storage (DIS) TLD (Thermoluminescent OSL (Optically EPDs (Electronic Descriptions technology to measure ionizing radiation through Dosimeter) measures Stimulated Personal Dosimeter) interactions that take place between the non- ionizing radiation Luminescence or APDs (Active volatile analog memory cell, which is surrounded exposure by assessing Dosimeter) measures Personal Dosimeter) by a gas filled ion chamber with a floating gate the intensity of visible ionizing radiation makes use of a diode that creates an electric charge enabling ionized light emitted by a crystal exposure when radiation (silicon or PIN, etc.) to particles to be measured by the change in the inside the detector when energy deposited in the detect “charges” induced electric charge created. -

Site-Specific Calibration of the Hanford Personnel Neutron Dosimeter
Gw^HI0n--7 PNL-SA-24010 SITE-SPECIFIC CALIBRATION OF THE HANFORD PERSONNEL NEUTRON DOSIMETER BEC 2 7 mm A. W. Endres Q o 7- L. W. Brackenbush ° f W. V. Baumgartner B. A. Rathbone October 1994 Presented at the Fourth Conference on Radiation Protection and Dosimetry October 23-27, 1994 Orlando, Florida Prepared for the U.S. Department of Energy under Contract DE-AC06-76RLO 1830 Pacific Northwest Laboratory Richland, Washington 99352 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsi• bility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Refer• ence herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recom• mendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. MUM1TED 4STR1BUTION Or TH« DU^- to u G£ DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. SITE-SPECIFIC CALIBRATION OF THE HANFORD PERSONNEL NEUTRON DOSIMETER A. W. Endres, L. W. Brackenbush, W. V. Baumgartner, and B. A. Rathbone Pacific Northwest Laboratory, Richland, Washington 99352 INTRODUCTION A new personnel dosimetry system, employing a standard Hanford thermo• luminescent dosimeter (TLD) and a combination dosimeter with both CR-39 nuclear track and TLD-albedo elements, is being implemented at Hanford. -

Radiation Glossary
Radiation Glossary Activity The rate of disintegration (transformation) or decay of radioactive material. The units of activity are Curie (Ci) and the Becquerel (Bq). Agreement State Any state with which the U.S. Nuclear Regulatory Commission has entered into an effective agreement under subsection 274b. of the Atomic Energy Act of 1954, as amended. Under the agreement, the state regulates the use of by-product, source, and small quantities of special nuclear material within said state. Airborne Radioactive Material Radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases. ALARA Acronym for "As Low As Reasonably Achievable". Making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken. It takes into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, societal and socioeconomic considerations, and in relation to utilization of radioactive materials and licensed materials in the public interest. Alpha Particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, with a mass number of 4 and a charge of +2. Annual Limit on Intake (ALI) Annual intake of a given radionuclide by "Reference Man" which would result in either a committed effective dose equivalent of 5 rems or a committed dose equivalent of 50 rems to an organ or tissue. Attenuation The process by which radiation is reduced in intensity when passing through some material. -

External and Internal Dosimetry
Chapter 7 External and Internal Dosimetry H-117 – Introductory Health Physics Slide 1 Objectives ¾ Discuss factors influencing external and internal doses ¾ Define terms used in external dosimetry ¾ Discuss external dosimeters such as TLDs, film badges, OSL dosimeters, pocket chambers, and electronic dosimetry H-117 – Introductory Health Physics Slide 2 Objectives ¾ Define terms used in internal dosimetry ¾ Discuss dose units and limits ¾ Define the ALI, DAC and DAC-hr ¾ Discuss radiation signs and postings H-117 – Introductory Health Physics Slide 3 Objectives ¾ Discuss types of bioassays ¾ Describe internal dose measuring equipment and facilities ¾ Discuss principles of internal dose calculation and work sample problems H-117 – Introductory Health Physics Slide 4 External Dosimetry H-117 – Introductory Health Physics Slide 5 External Dosimetry Gamma, beta or neutron radiation emitted by radioactive material outside the body irradiates the skin, lens of the eye, extremities & the whole body (i.e. internal organs) H-117 – Introductory Health Physics Slide 6 External Dosimetry (cont.) ¾ Alpha particles cannot penetrate the dead layer of skin (0.007 cm) ¾ Beta particles are primarily a skin hazard. However, energetic betas can penetrate the lens of an eye (0.3 cm) and deeper tissue (1 cm) ¾ Beta sources can produce more penetrating radiation through bremsstrahlung ¾ Primary sources of external exposure are photons and neutrons ¾ External dose must be measured by means of appropriate dosimeters H-117 – Introductory Health Physics Slide 7 -

TLD) Service PROTECT YOURSELF and YOUR EMPLOYEES!
Thermoluminescent Dosimetry (TLD) Service PROTECT YOURSELF AND YOUR EMPLOYEES! About Us PL Medical Co., LLC, offers TLD dosimeters which are based on state-of-the-art technology. PL Medical Co., LLC supplies accredited dosimetry services to industries in which radiation monitoring is desired or mandated by state and/or federal regulatory standards. PL Medical Co., LLC is accredited to provide dosimetry services by the National Voluntary Laboratory Accreditation Program (NVLAP), a division of National Institute of Standards and Technology (NIST). Advantages • Access to myTLDaccount • No account maintenance or set-up fees Applications • One control badge provided free of charge • Primary Care • Flexibility of the wear period •EMS • Automatic dosimeter replacement every three months •Podiatry for one year • Occupational Health • A reply envelope to return badges • Corrections • Computer generated reports sent to the customer • Orthopedics within 2-3 weeks of receiving the dosimeters (exposure • Homeland Security history automatically updated) • X-Ray Technicians • Advance notice not required for cancellation. Prompt •Hospitals credit for termination of service of an employee • Universities and National Laboratories • Each dose report is reviewed to check if it meets • Dentists ALARA limits •Chiropractors • Customer care – you will always be in contact with a • Veterinary “live person” Logistics of Service Product Description • An order is placed at http://manageTLD.plmedical.com • Possible wear period of up to twelve months or by phoning, -

11. Dosimetry Fundamentals
Outline • Introduction Dosimetry Fundamentals • Dosimeter model • Interpretation of dosimeter measurements Chapter 11 – Photons and neutrons – Charged particles • General characteristics of dosimeters F.A. Attix, Introduction to Radiological Physics and Radiation Dosimetry • Summary Introduction Dosimeter • Radiation dosimetry deals with the determination • A dosimeter can be generally defined as (i.e., by measurement or calculation) of the any device that is capable of providing a absorbed dose or dose rate resulting from the interaction of ionizing radiation with matter reading R that is a measure of the absorbed • Other radiologically relevant quantities are dose Dg deposited in its sensitive volume V exposure, kerma, fluence, dose equivalent, energy by ionizing radiation imparted, etc. can be determined • If the dose is not homogeneous • Measuring one quantity (usually the absorbed dose) another one can be derived through throughout the sensitive calculations based on the defined relationships volume, then R is a measure of mean value Dg Dosimeter Simple dosimeter model • Ordinarily one is not interested in measuring • A dosimeter can generally be considered as the absorbed dose in a dosimeter’s sensitive consisting of a sensitive volume V filled with a volume itself, but rather as a means of medium g, surrounded by a wall (or envelope, determining the dose (or a related quantity) for container, etc.) of another medium w having a another medium in which direct measurements thickness t 0 are not feasible • A simple dosimeter can be -

Radiological Information
RADIOLOGICAL INFORMATION Frequently Asked Questions Radiation Information A. Radiation Basics 1. What is radiation? Radiation is a form of energy. It is all around us. It is a type of energy in the form of particles or electromagnetic rays that are given off by atoms. The type of radiation we are concerned with, during radiation incidents, is “ionizing radiation”. Radiation is colorless, odorless, tasteless, and invisible. 2. What is radioactivity? It is the process of emission of radiation from a material. 3. What is ionizing radiation? It is a type of radiation that has enough energy to break chemical bonds (knocking out electrons). 4. What is non-ionizing radiation? Non-ionizing radiation is a type of radiation that has a long wavelength. Long wavelength radiations do not have enough energy to "ionize" materials (knock out electrons). Some types of non-ionizing radiation sources include radio waves, microwaves produced by cellular phones, microwaves from microwave ovens and radiation given off by television sets. 5. What types of ionizing radiation are there? Three different kinds of ionizing radiation are emitted from radioactive materials: alpha (helium nuclei); beta (usually electrons); x-rays; and gamma (high energy, short wave length light). • Alpha particles stop in a few inches of air, or a thin sheet of cloth or even paper. Alpha emitting materials pose serious health dangers primarily if they are inhaled. • Beta particles are easily stopped by aluminum foil or human skin. Unless Beta particles are ingested or inhaled they usually pose little danger to people. • Gamma photons/rays and x-rays are very penetrating. -

Area Monitoring Dosimeter Program for the Pacific Northwest National Laboratory: Results for CY 2000
PNNL-13604 Area Monitoring Dosimeter Program for the Pacific Northwest National Laboratory: Results for CY 2000 S. R. Bivins G. A. Stoetzel July 2001 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RL01830 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor Battelle Memorial Institute, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or Battelle Memorial Institute. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. PACIFIC NORTHWEST NATIONAL LABORATORY operated by BATTELLE for the UNITED STATES DEPARTMENT OF ENERGY under Contract DE-AC06-76RL01830 Printed in the United States of America Available to DOE and DOE contractors from the Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062; ph: (865) 576-8401 fax: (865) 576-5728 email: [email protected] Available to the public from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Rd., Springfield, VA 22161 ph: (800) 553-6847 fax: (703) 605-6900 email: [email protected] online ordering: http://www.ntis.gov/ordering.htm This document was printed on recycled paper. -

US Dod Comprehensive CBRN/Trauma Treatment Program
US DoD Comprehensive CBRN/trauma treatment program U.S. Army Institute of Surgical Research COL Andrew P. Cap, MS, MD, PhD, FACP Chief, Coagulation and Blood Research Program, USAISR Program Director, SAMMC Clinical Research Fellowship Associate Professor of Medicine, Uniformed Services University Adjunct Associate Professor, University of Texas Hematology-Oncology Consultant to the Army Surgeon General Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Air Force or the Department of Defense. Disclosures: The authors have no relevant conflicts of interest. Acknowledgement: Mary Homer, PhD Ashley Cecere, MS BARDA (HHS) & DoD Collaboration The Challenge: Surviving in a CBRN Environment • “The Cold War called and it wants its medical planning back.” • Recent events: this is real! – Nerve agent, chlorine in Syria – DPRK scenario? Iran? – Terrorism? – Others? • US Military, NATO, civilians, etc. multiple missions – Major combat operations in contaminated environment – SOF missions (counter-terrorism, weapon seizure/destruction, secure nuclear fuel cycle facility or chemical plant or biological lab) – Homeland defense, react to event Trauma + CBRN? • We are talking about guns + explosives + CBRN trauma is going to happen. A lot. • Current CBRN doctrine: – Stove-piped by class of exposure (understandable) – Focus on decontamination, supportive care – Completely disconnected from resuscitation, trauma care • Current trauma + CBRN management: – Up-code triage of any patient with CBRN exposure And that’s about it… Trauma + CBRN issues • Lethality of trauma + lethality of agent Recent small exercises: • Delay in care for decontamination: -- Mountain Path (3SFG) – Patient movement -- RDCR PreConf – Team division (hot zone vs. -

Dosimetry System 1) When Calibrated After Every 10 Sv of Accumulated Dose 2) PTB Approved up to 7 Mev
Radiation. Safety. DIS-1 Direct Ion Storage Dosimeter Nuclear Homeland Industrial and Healthcare Labs and Power Security Manufacturing Education & Defense OVERVIEW KEY FEATURES The RADOS DIS-1 personal dosimeter is based • Direct measurement of Hp(10) and Hp(0.07) on an ionization chamber combined with a over the entire energy range modern electronic Direct Ion Storage (DIS) • Instant readout memory cell. The Ion Chamber is widely used • Extremely easy readout process as a reference detector in radiation detection • Passive operation and is now available in everyday dosimetry • Not sensitive to EM and RF interferences applications. • Operation at high dose rates • Operation at pulsed fields The DIS-1 dosimeter could be described as a • Complies with: IEC 61066, 62387:2012, passive electronic TLD or Film badge, which 17025:2005, PTB can be read instantly and non-destructively without any loss of dose information. This unique feature allows the user of the DIS-1 to read his/her accumulated doses whenever necessary. The DIS-1 dosimeter has a small, lightweight, rugged and watertight construction, which makes the DIS-1 dosimeter reliable and easy to use. The radiological range of the DIS-1 covers the entire Hp(10) and Hp(0.07) photon and beta energies without any compromises. Health Physics www.mirion.com DIS-1 I Direct Ion Storage Dosimeter PHYSICAL CHARACTERISTICS ENVIRONMENTAL CHARACTERISTICS • Detector type: three TMDIS (Direct Ion Storage) • Temperature range from - 10°C to +50°C (14°F to 122°F) detectors and two MOSFET detectors • Enclosure