IQ-TREE Version 2.1.2: Tutorials and Manual Phylogenomic Software by Maximum Likelihood
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 A) Login to the System B) Use the Appropriate Command to Determine Your Login Shell C) Use the /Etc/Passwd File to Verify the Result of Step B
CSE ([email protected] II-Sem) EXP-3 1 a) Login to the system b) Use the appropriate command to determine your login shell c) Use the /etc/passwd file to verify the result of step b. d) Use the ‘who’ command and redirect the result to a file called myfile1. Use the more command to see the contents of myfile1. e) Use the date and who commands in sequence (in one line) such that the output of date will display on the screen and the output of who will be redirected to a file called myfile2. Use the more command to check the contents of myfile2. 2 a) Write a “sed” command that deletes the first character in each line in a file. b) Write a “sed” command that deletes the character before the last character in each line in a file. c) Write a “sed” command that swaps the first and second words in each line in a file. a. Log into the system When we return on the system one screen will appear. In this we have to type 100.0.0.9 then we enter into editor. It asks our details such as Login : krishnasai password: Then we get log into the commands. bphanikrishna.wordpress.com FOSS-LAB Page 1 of 10 CSE ([email protected] II-Sem) EXP-3 b. use the appropriate command to determine your login shell Syntax: $ echo $SHELL Output: $ echo $SHELL /bin/bash Description:- What is "the shell"? Shell is a program that takes your commands from the keyboard and gives them to the operating system to perform. -
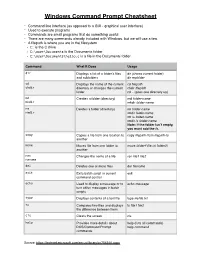
Windows Command Prompt Cheatsheet
Windows Command Prompt Cheatsheet - Command line interface (as opposed to a GUI - graphical user interface) - Used to execute programs - Commands are small programs that do something useful - There are many commands already included with Windows, but we will use a few. - A filepath is where you are in the filesystem • C: is the C drive • C:\user\Documents is the Documents folder • C:\user\Documents\hello.c is a file in the Documents folder Command What it Does Usage dir Displays a list of a folder’s files dir (shows current folder) and subfolders dir myfolder cd Displays the name of the current cd filepath chdir directory or changes the current chdir filepath folder. cd .. (goes one directory up) md Creates a folder (directory) md folder-name mkdir mkdir folder-name rm Deletes a folder (directory) rm folder-name rmdir rmdir folder-name rm /s folder-name rmdir /s folder-name Note: if the folder isn’t empty, you must add the /s. copy Copies a file from one location to copy filepath-from filepath-to another move Moves file from one folder to move folder1\file.txt folder2\ another ren Changes the name of a file ren file1 file2 rename del Deletes one or more files del filename exit Exits batch script or current exit command control echo Used to display a message or to echo message turn off/on messages in batch scripts type Displays contents of a text file type myfile.txt fc Compares two files and displays fc file1 file2 the difference between them cls Clears the screen cls help Provides more details about help (lists all commands) DOS/Command Prompt help command commands Source: https://technet.microsoft.com/en-us/library/cc754340.aspx. -

Don't Trust Traceroute (Completely)
Don’t Trust Traceroute (Completely) Pietro Marchetta, Valerio Persico, Ethan Katz-Bassett Antonio Pescapé University of Southern California, CA, USA University of Napoli Federico II, Italy [email protected] {pietro.marchetta,valerio.persico,pescape}@unina.it ABSTRACT In this work, we propose a methodology based on the alias resolu- tion process to demonstrate that the IP level view of the route pro- vided by traceroute may be a poor representation of the real router- level route followed by the traffic. More precisely, we show how the traceroute output can lead one to (i) inaccurately reconstruct the route by overestimating the load balancers along the paths toward the destination and (ii) erroneously infer routing changes. Categories and Subject Descriptors C.2.1 [Computer-communication networks]: Network Architec- ture and Design—Network topology (a) Traceroute reports two addresses at the 8-th hop. The common interpretation is that the 7-th hop is splitting the traffic along two Keywords different forwarding paths (case 1); another explanation is that the 8- th hop is an RFC compliant router using multiple interfaces to reply Internet topology; Traceroute; IP alias resolution; IP to Router to the source (case 2). mapping 1 1. INTRODUCTION 0.8 Operators and researchers rely on traceroute to measure routes and they assume that, if traceroute returns different IPs at a given 0.6 hop, it indicates different paths. However, this is not always the case. Although state-of-the-art implementations of traceroute al- 0.4 low to trace all the paths -

“Log” File in Stata
Updated July 2018 Creating a “Log” File in Stata This set of notes describes how to create a log file within the computer program Stata. It assumes that you have set Stata up on your computer (see the “Getting Started with Stata” handout), and that you have read in the set of data that you want to analyze (see the “Reading in Stata Format (.dta) Data Files” handout). A log file records all your Stata commands and output in a given session, with the exception of graphs. It is usually wise to retain a copy of the work that you’ve done on a given project, to refer to while you are writing up your findings, or later on when you are revising a paper. A log file is a separate file that has either a “.log” or “.smcl” extension. Saving the log as a .smcl file (“Stata Markup and Control Language file”) keeps the formatting from the Results window. It is recommended to save the log as a .log file. Although saving it as a .log file removes the formatting and saves the output in plain text format, it can be opened in most text editing programs. A .smcl file can only be opened in Stata. To create a log file: You may create a log file by typing log using ”filepath & filename” in the Stata Command box. On a PC: If one wanted to save a log file (.log) for a set of analyses on hard disk C:, in the folder “LOGS”, one would type log using "C:\LOGS\analysis_log.log" On a Mac: If one wanted to save a log file (.log) for a set of analyses in user1’s folder on the hard drive, in the folder “logs”, one would type log using "/Users/user1/logs/analysis_log.log" If you would like to replace an existing log file with a newer version add “replace” after the file name (Note: PC file path) log using "C:\LOGS\analysis_log.log", replace Alternately, you can use the menu: click on File, then on Log, then on Begin. -

Basic STATA Commands
Summer School on Capability and Multidimensional Poverty OPHI-HDCA, 2011 Oxford Poverty and Human Development Initiative (OPHI) http://ophi.qeh.ox.ac.uk Oxford Dept of International Development, Queen Elizabeth House, University of Oxford Basic STATA commands Typical STATA window Review Results Variables Commands Exploring your data Create a do file doedit Change your directory cd “c:\your directory” Open your database use database, clear Import from Excel (csv file) insheet using "filename.csv" Condition (after the following commands) if var1==3 or if var1==”male” Equivalence symbols: == equal; ~= not equal; != not equal; > greater than; >= greater than or equal; < less than; <= less than or equal; & and; | or. Weight [iw=weight] or [aw=weight] Browse your database browse Look for a variables lookfor “any word/topic” Summarize a variable (mean, standard su variable1 variable2 variable3 deviation, min. and max.) Tabulate a variable (per category) tab variable1 (add a second variables for cross tabs) Statistics for variables by subgroups tabstat variable1 variable2, s(n mean) by(group) Information of a variable (coding) codebook variable1, tab(99) Keep certain variables (use drop for the keep var1 var2 var3 opposite) Save a dataset save filename, [replace] Summer School on Capability and Multidimensional Poverty OPHI-HDCA, 2011 Creating Variables Generate a new variable (a number or a gen new_variable = 1 combinations of other variables) gen new_variable = variable1+ variable2 Generate a new variable conditional gen new_variable -

Forest Quickstart Guide for Linguists
Forest Quickstart Guide for Linguists Guido Vanden Wyngaerd [email protected] June 28, 2020 Contents 1 Introduction 1 2 Loading Forest 2 3 Basic Usage 2 4 Adjusting node spacing 4 5 Triangles 7 6 Unlabelled nodes 9 7 Horizontal alignment of terminals 10 8 Arrows 11 9 Highlighting 14 1 Introduction Forest is a package for drawing linguistic (and other) tree diagrams de- veloped by Sašo Živanović. This manual provides a quickstart guide for linguists with just the essential things that you need to get started. More 1 extensive documentation is available from the CTAN-archive. Forest is based on the TikZ package; more information about its commands, in par- ticular those controlling the appearance of the nodes, the arrows, and the highlighting can be found in the TikZ documentation. 2 Loading Forest In your preamble, put \usepackage[linguistics]{forest} The linguistics option makes for nice trees, in which the branches meet above the two nodes that they join; it will also align the example number (provided by linguex) with the top of the tree: (1) CP C IP I VP V NP 3 Basic Usage Forest uses a familiar labelled brackets syntax. The code below will out- put the tree in (1) above (\ex. requires the linguex package and provides the example number): \ex. \begin{forest} [CP[C][IP[I][VP[V][NP]]]] \end{forest} Forest will parse the above code without problem, but you are likely to soon get lost in your labelled brackets with more complicated trees if you write the code this way. The better alternative is to arrange the nodes over multiple lines: 2 \ex. -

Treebase: an R Package for Discovery, Access and Manipulation of Online Phylogenies
Methods in Ecology and Evolution 2012, 3, 1060–1066 doi: 10.1111/j.2041-210X.2012.00247.x APPLICATION Treebase: an R package for discovery, access and manipulation of online phylogenies Carl Boettiger1* and Duncan Temple Lang2 1Center for Population Biology, University of California, Davis, CA,95616,USA; and 2Department of Statistics, University of California, Davis, CA,95616,USA Summary 1. The TreeBASE portal is an important and rapidly growing repository of phylogenetic data. The R statistical environment has also become a primary tool for applied phylogenetic analyses across a range of questions, from comparative evolution to community ecology to conservation planning. 2. We have developed treebase, an open-source software package (freely available from http://cran.r-project. org/web/packages/treebase) for the R programming environment, providing simplified, programmatic and inter- active access to phylogenetic data in the TreeBASE repository. 3. We illustrate how this package creates a bridge between the TreeBASE repository and the rapidly growing collection of R packages for phylogenetics that can reduce barriers to discovery and integration across phyloge- netic research. 4. We show how the treebase package can be used to facilitate replication of previous studies and testing of methods and hypotheses across a large sample of phylogenies, which may help make such important reproduc- ibility practices more common. Key-words: application programming interface, database, programmatic, R, software, TreeBASE, workflow include, but are not limited to, ancestral state reconstruction Introduction (Paradis 2004; Butler & King 2004), diversification analysis Applications that use phylogenetic information as part of their (Paradis 2004; Rabosky 2006; Harmon et al. -

What Is UNIX? the Directory Structure Basic Commands Find
What is UNIX? UNIX is an operating system like Windows on our computers. By operating system, we mean the suite of programs which make the computer work. It is a stable, multi-user, multi-tasking system for servers, desktops and laptops. The Directory Structure All the files are grouped together in the directory structure. The file-system is arranged in a hierarchical structure, like an inverted tree. The top of the hierarchy is traditionally called root (written as a slash / ) Basic commands When you first login, your current working directory is your home directory. In UNIX (.) means the current directory and (..) means the parent of the current directory. find command The find command is used to locate files on a Unix or Linux system. find will search any set of directories you specify for files that match the supplied search criteria. The syntax looks like this: find where-to-look criteria what-to-do All arguments to find are optional, and there are defaults for all parts. where-to-look defaults to . (that is, the current working directory), criteria defaults to none (that is, select all files), and what-to-do (known as the find action) defaults to ‑print (that is, display the names of found files to standard output). Examples: find . –name *.txt (finds all the files ending with txt in current directory and subdirectories) find . -mtime 1 (find all the files modified exact 1 day) find . -mtime -1 (find all the files modified less than 1 day) find . -mtime +1 (find all the files modified more than 1 day) find . -

NASP: an Accurate, Rapid Method for the Identification of Snps in WGS Datasets That Supports Flexible Input and Output Formats Jason Sahl
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Environmental and Occupational Health Faculty Environmental and Occupational Health Publications 8-2016 NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats Jason Sahl Darrin Lemmer Jason Travis James Schupp John Gillece See next page for additional authors Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/sphhs_enviro_facpubs Part of the Bioinformatics Commons, Integrative Biology Commons, Research Methods in Life Sciences Commons, and the Systems Biology Commons APA Citation Sahl, J., Lemmer, D., Travis, J., Schupp, J., Gillece, J., Aziz, M., & +several additional authors (2016). NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats. Microbial Genomics, 2 (8). http://dx.doi.org/10.1099/mgen.0.000074 This Journal Article is brought to you for free and open access by the Environmental and Occupational Health at Health Sciences Research Commons. It has been accepted for inclusion in Environmental and Occupational Health Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Authors Jason Sahl, Darrin Lemmer, Jason Travis, James Schupp, John Gillece, Maliha Aziz, and +several additional authors This journal article is available at Health Sciences Research Commons: http://hsrc.himmelfarb.gwu.edu/sphhs_enviro_facpubs/212 Methods Paper NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats Jason W. Sahl,1,2† Darrin Lemmer,1† Jason Travis,1 James M. -

PC23 and PC43 Desktop Printer User Manual Document Change Record This Page Records Changes to This Document
PC23 | PC43 Desktop Printer PC23d, PC43d, PC43t User Manual Intermec by Honeywell 6001 36th Ave.W. Everett, WA 98203 U.S.A. www.intermec.com The information contained herein is provided solely for the purpose of allowing customers to operate and service Intermec-manufactured equipment and is not to be released, reproduced, or used for any other purpose without written permission of Intermec by Honeywell. Information and specifications contained in this document are subject to change without prior notice and do not represent a commitment on the part of Intermec by Honeywell. © 2012–2014 Intermec by Honeywell. All rights reserved. The word Intermec, the Intermec logo, Fingerprint, Ready-to-Work, and SmartSystems are either trademarks or registered trademarks of Intermec by Honeywell. For patent information, please refer to www.hsmpats.com Wi-Fi is a registered certification mark of the Wi-Fi Alliance. Microsoft, Windows, and the Windows logo are registered trademarks of Microsoft Corporation in the United States and/or other countries. Bluetooth is a trademark of Bluetooth SIG, Inc., U.S.A. The products described herein comply with the requirements of the ENERGY STAR. As an ENERGY STAR partner, Intermec Technologies has determined that this product meets the ENERGY STAR guidelines for energy efficiency. For more information on the ENERGY STAR program, see www.energystar.gov. The ENERGY STAR does not represent EPA endorsement of any product or service. ii PC23 and PC43 Desktop Printer User Manual Document Change Record This page records changes to this document. The document was originally released as Revision 001. Version Number Date Description of Change 005 12/2014 Revised to support MR7 firmware release. -

CS101 Lecture 8
What is a program? What is a “Window Manager” ? What is a “GUI” ? How do you navigate the Unix directory tree? What is a wildcard? Readings: See CCSO’s Unix pages and 8-2 Applications Unix Engineering Workstations UNIX- operating system / C- programming language / Matlab Facilitate machine independent program development Computer program(software): a sequence of instructions that tells the computer what to do. A program takes raw data as input and produces information as output. System Software: • Operating Systems Unix,Windows,MacOS,VM/CMS,... • Editor Programs gedit, pico, vi Applications Software: • Translators and Interpreters gcc--gnu c compiler matlab – interpreter • User created Programs!!! 8-4 X-windows-a Window Manager and GUI(Graphical User Interface) Click Applications and follow the menus or click on an icon to run a program. Click Terminal to produce a command line interface. When the following slides refer to Unix commands it is assumed that these are entered on the command line that begins with the symbol “>” (prompt symbol). Data, information, computer instructions, etc. are saved in secondary storage (auxiliary storage) in files. Files are collected or organized in directories. Each user on a multi-user system has his/her own home directory. In Unix users and system administrators organize files and directories in a hierarchical tree. secondary storage Home Directory When a user first logs in on a computer, the user will be “in” the users home directory. To find the name of the directory type > pwd (this is an acronym for print working directory) So the user is in the directory “gambill” The string “/home/gambill” is called a path. -

Trees, Binary Search Trees, Heaps & Applications Dr. Chris Bourke
Trees Trees, Binary Search Trees, Heaps & Applications Dr. Chris Bourke Department of Computer Science & Engineering University of Nebraska|Lincoln Lincoln, NE 68588, USA [email protected] http://cse.unl.edu/~cbourke 2015/01/31 21:05:31 Abstract These are lecture notes used in CSCE 156 (Computer Science II), CSCE 235 (Dis- crete Structures) and CSCE 310 (Data Structures & Algorithms) at the University of Nebraska|Lincoln. This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License 1 Contents I Trees4 1 Introduction4 2 Definitions & Terminology5 3 Tree Traversal7 3.1 Preorder Traversal................................7 3.2 Inorder Traversal.................................7 3.3 Postorder Traversal................................7 3.4 Breadth-First Search Traversal..........................8 3.5 Implementations & Data Structures.......................8 3.5.1 Preorder Implementations........................8 3.5.2 Inorder Implementation.........................9 3.5.3 Postorder Implementation........................ 10 3.5.4 BFS Implementation........................... 12 3.5.5 Tree Walk Implementations....................... 12 3.6 Operations..................................... 12 4 Binary Search Trees 14 4.1 Basic Operations................................. 15 5 Balanced Binary Search Trees 17 5.1 2-3 Trees...................................... 17 5.2 AVL Trees..................................... 17 5.3 Red-Black Trees.................................. 19 6 Optimal Binary Search Trees 19 7 Heaps 19