COG7 Deficiency in Drosophila Generates Multifaceted
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seq2pathway Vignette
seq2pathway Vignette Bin Wang, Xinan Holly Yang, Arjun Kinstlick May 19, 2021 Contents 1 Abstract 1 2 Package Installation 2 3 runseq2pathway 2 4 Two main functions 3 4.1 seq2gene . .3 4.1.1 seq2gene flowchart . .3 4.1.2 runseq2gene inputs/parameters . .5 4.1.3 runseq2gene outputs . .8 4.2 gene2pathway . 10 4.2.1 gene2pathway flowchart . 11 4.2.2 gene2pathway test inputs/parameters . 11 4.2.3 gene2pathway test outputs . 12 5 Examples 13 5.1 ChIP-seq data analysis . 13 5.1.1 Map ChIP-seq enriched peaks to genes using runseq2gene .................... 13 5.1.2 Discover enriched GO terms using gene2pathway_test with gene scores . 15 5.1.3 Discover enriched GO terms using Fisher's Exact test without gene scores . 17 5.1.4 Add description for genes . 20 5.2 RNA-seq data analysis . 20 6 R environment session 23 1 Abstract Seq2pathway is a novel computational tool to analyze functional gene-sets (including signaling pathways) using variable next-generation sequencing data[1]. Integral to this tool are the \seq2gene" and \gene2pathway" components in series that infer a quantitative pathway-level profile for each sample. The seq2gene function assigns phenotype-associated significance of genomic regions to gene-level scores, where the significance could be p-values of SNPs or point mutations, protein-binding affinity, or transcriptional expression level. The seq2gene function has the feasibility to assign non-exon regions to a range of neighboring genes besides the nearest one, thus facilitating the study of functional non-coding elements[2]. Then the gene2pathway summarizes gene-level measurements to pathway-level scores, comparing the quantity of significance for gene members within a pathway with those outside a pathway. -

Autism Multiplex Family with 16P11.2P12.2 Microduplication Syndrome in Monozygotic Twins and Distal 16P11.2 Deletion in Their Brother
European Journal of Human Genetics (2012) 20, 540–546 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother Anne-Claude Tabet1,2,3,4, Marion Pilorge2,3,4, Richard Delorme5,6,Fre´de´rique Amsellem5,6, Jean-Marc Pinard7, Marion Leboyer6,8,9, Alain Verloes10, Brigitte Benzacken1,11,12 and Catalina Betancur*,2,3,4 The pericentromeric region of chromosome 16p is rich in segmental duplications that predispose to rearrangements through non-allelic homologous recombination. Several recurrent copy number variations have been described recently in chromosome 16p. 16p11.2 rearrangements (29.5–30.1 Mb) are associated with autism, intellectual disability (ID) and other neurodevelopmental disorders. Another recognizable but less common microdeletion syndrome in 16p11.2p12.2 (21.4 to 28.5–30.1 Mb) has been described in six individuals with ID, whereas apparently reciprocal duplications, studied by standard cytogenetic and fluorescence in situ hybridization techniques, have been reported in three patients with autism spectrum disorders. Here, we report a multiplex family with three boys affected with autism, including two monozygotic twins carrying a de novo 16p11.2p12.2 duplication of 8.95 Mb (21.28–30.23 Mb) characterized by single-nucleotide polymorphism array, encompassing both the 16p11.2 and 16p11.2p12.2 regions. The twins exhibited autism, severe ID, and dysmorphic features, including a triangular face, deep-set eyes, large and prominent nasal bridge, and tall, slender build. The eldest brother presented with autism, mild ID, early-onset obesity and normal craniofacial features, and carried a smaller, overlapping 16p11.2 microdeletion of 847 kb (28.40–29.25 Mb), inherited from his apparently healthy father. -

Linkage Analysis in 15 Families, Physical And
European Journal of Human Genetics (2002) 11, 145 – 154 ª 2002 Nature Publishing Group All rights reserved 1018 – 4813/02 $25.00 www.nature.com/ejhg ARTICLE Familial juvenile hyperuricaemic nephropathy (FJHN): linkage analysis in 15 families, physical and transcriptional characterisation of the FJHN critical region on chromosome 16p11.2 and the analysis of seven candidate genes Blanka Stibu˚ rkova´1, Jacek Majewski2, Katerˇina Hodanˇova´1, Lenka Ondrova´1, Marke´ta Jerˇa´bkova´1, Marie Zika´nova´1, Petr Vylet’al1, Ivan Sˇebesta3, Anthony Marinaki4, Anne Simmonds4, Gert Matthijs5, Jean-Pierre Fryns5, Rosa Torres6, Juan Garcı´a Puig7, Jurg Ott2 and Stanislav Kmoch*,1 1Center for Integrated Genomics, Institute for Inherited Metabolic Disorders, Charles University 1st School of Medicine and General Faculty Hospital Prague, Czech Republic; 2Laboratory of Statistical Genetics, Rockefeller University, New York, NY, USA; 3Department of Clinical Biochemistry, Charles University 1st School of Medicine and General Faculty Hospital Prague, Czech Republic; 4Purine Research Unit, GKT, Guy’s Hospital, London, UK; 5Center for Human Genetics, University of Leuven, Leuven, Belgium; 6Department of Clinical Biochemistry, La Paz Hospital, Madrid, Spain; 7Division of Internal Medicine, La Paz Hospital, Madrid, Spain Familial juvenile hyperuricaemic nephropathy (FJHN) is an autosomal dominant renal disease characterised by juvenile onset of hyperuricaemia, gouty arthritis, and progressive renal failure at an early age. Recent studies in four kindreds showed linkage of a gene for FJHN to the same genomic interval on chromosome 16p11.2, where the gene for the phenotypically similar medullary cystic disease type 2 (MCKD2) has been localised. In this study we performed linkage analysis in additional 15 FJHN families. -

Congenital Disorders of Glycosylation from a Neurological Perspective
brain sciences Review Congenital Disorders of Glycosylation from a Neurological Perspective Justyna Paprocka 1,* , Aleksandra Jezela-Stanek 2 , Anna Tylki-Szyma´nska 3 and Stephanie Grunewald 4 1 Department of Pediatric Neurology, Faculty of Medical Science in Katowice, Medical University of Silesia, 40-752 Katowice, Poland 2 Department of Genetics and Clinical Immunology, National Institute of Tuberculosis and Lung Diseases, 01-138 Warsaw, Poland; [email protected] 3 Department of Pediatrics, Nutrition and Metabolic Diseases, The Children’s Memorial Health Institute, W 04-730 Warsaw, Poland; [email protected] 4 NIHR Biomedical Research Center (BRC), Metabolic Unit, Great Ormond Street Hospital and Institute of Child Health, University College London, London SE1 9RT, UK; [email protected] * Correspondence: [email protected]; Tel.: +48-606-415-888 Abstract: Most plasma proteins, cell membrane proteins and other proteins are glycoproteins with sugar chains attached to the polypeptide-glycans. Glycosylation is the main element of the post- translational transformation of most human proteins. Since glycosylation processes are necessary for many different biological processes, patients present a diverse spectrum of phenotypes and severity of symptoms. The most frequently observed neurological symptoms in congenital disorders of glycosylation (CDG) are: epilepsy, intellectual disability, myopathies, neuropathies and stroke-like episodes. Epilepsy is seen in many CDG subtypes and particularly present in the case of mutations -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -
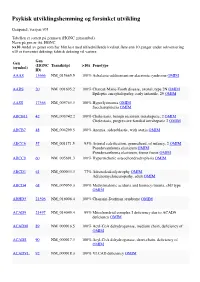
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Targeted Polymerase Chain Reaction-Based Enrichment and Next Generation Sequencing for Diagnostic Testing of Congenital Disorders of Glycosylation Melanie A
ARTICLE Targeted polymerase chain reaction-based enrichment and next generation sequencing for diagnostic testing of congenital disorders of glycosylation Melanie A. Jones, PhD1, Shruti Bhide, MS1, Ephrem Chin, MB(ASCP), QLC1, Bobby G. Ng, BS2, Devin Rhodenizer, BS1, Victor W. Zhang, MD, PhD1, Jessica J. Sun, MS1, Alice Tanner, PhD, MS1, Hudson H. Freeze, PhD2, and Madhuri R. Hegde, PhD, FACMG1 2 Purpose: Congenital disorders of glycosylation are a heterogeneous stability and in the immune response ; hence, the proper devel- group of disorders caused by deficient glycosylation, primarily affecting opment and functioning of many organ systems depend on the N-linked pathway. It is estimated that more than 40% of congenital normal N-glycosylation. Deficient N-glycosylation results in 3 disorders of glycosylation patients lack a confirmatory molecular multiple organ dysfunction that can be life threatening. Con- diagnosis. The purpose of this study was to improve molecular genital disorders of glycosylation (CDG) are a group of more diagnosis for congenital disorders of glycosylation by developing than 30 autosomal recessive disorders caused by deficient gly- 4 and validating a next generation sequencing panel for comprehensive cosylation, primarily affecting the N-linked pathway. Symp- mutation detection in 24 genes known to cause congenital disorders toms of CDG can include severe developmental delay, ataxia, of glycosylation. Methods: Next generation sequencing validation seizures, liver fibrosis, retinopathy, cardiac dysfunction, and 3,5 was performed on 12 positive control congenital disorders of gly- coagulopathies. CDG occurs worldwide, with an estimated 6 cosylation patients. These samples were blinded as to the disease- prevalence as high as 1 in 20,000. -

ARP55242 P050) Data Sheet
COG4 antibody - middle region (ARP55242_P050) Data Sheet Product Number ARP55242_P050 Product Name COG4 antibody - middle region (ARP55242_P050) Size 50ug Gene Symbol COG4 Alias Symbols COD1; DKFZp586E1519; CDG2J Nucleotide Accession# NM_015386 Protein Size (# AA) 789 amino acids Molecular Weight 89kDa Subunit 4 Product Format Lyophilized powder NCBI Gene Id 25839 Host Rabbit Clonality Polyclonal Official Gene Full Name Component of oligomeric golgi complex 4 Gene Family COG This is a rabbit polyclonal antibody against COG4. It was validated on Western Blot using a cell lysate as a Description positive control. Aviva Systems Biology strives to provide antibodies covering each member of a whole protein family of your interest. We also use our best efforts to provide you antibodies recognize various epitopes of a target protein. For availability of antibody needed for your experiment, please inquire (). Peptide Sequence Synthetic peptide located within the following region: LFSQGIGGEQAQAKFDSCLSDLAAVSNKFRDLLQEGLTELNSTAIKPQVQ Target Reference Suzuki,Y., (2004) Genome Res. 14 (9), 1711-1718 Multiprotein complexes are key determinants of Golgi apparatus structure and its capacity for intracellular transport and glycoprotein modification. Several complexes have been identified, including the Golgi transport complex (GTC), the LDLC complex, which is involved in glycosylation reactions, and the SEC34 complex, which is involved in vesicular transport. These 3 complexes are identical and have been termed the conserved oligomeric Golgi (COG) complex, which includes COG4.Multiprotein complexes are key determinants of Golgi Description of Target apparatus structure and its capacity for intracellular transport and glycoprotein modification. Several complexes have been identified, including the Golgi transport complex (GTC), the LDLC complex, which is involved in glycosylation reactions, and the SEC34 complex, which is involved in vesicular transport. -

Cog5–Cog7 Crystal Structure Reveals Interactions Essential for the Function of a Multisubunit Tethering Complex
Cog5–Cog7 crystal structure reveals interactions essential for the function of a multisubunit tethering complex Jun Yong Haa, Irina D. Pokrovskayab, Leslie K. Climerb, Gregory R. Shimamuraa, Tetyana Kudlykb, Philip D. Jeffreya, Vladimir V. Lupashinb,c, and Frederick M. Hughsona,1 aDepartment of Molecular Biology, Princeton University, Princeton, NJ 08544; bDepartment of Physiology and Biophysics, University of Arkansas for Medical Sciences, Little Rock, AR 72205; and cBiological Institute, Tomsk State University, Tomsk, 634050, Russian Federation Edited by Thomas C. Südhof, Stanford University School of Medicine, Stanford, CA, and approved September 30, 2014 (received for review August 4, 2014) The conserved oligomeric Golgi (COG) complex is required, along have been structurally characterized to date (11, 14). Defining the with SNARE and Sec1/Munc18 (SM) proteins, for vesicle docking quaternary structure of the other CATCHR-family MTCs remains and fusion at the Golgi. COG, like other multisubunit tethering a major challenge. complexes (MTCs), is thought to function as a scaffold and/or The COG complex is an MTC that is essential for vesicle chaperone to direct the assembly of productive SNARE complexes transport within the Golgi apparatus and from endosomal com- at the sites of membrane fusion. Reflecting this essential role, partments to the Golgi (3). Defects in individual COG subunits mutations in the COG complex can cause congenital disorders of can lead to the aberrant distribution of glycosylation enzymes glycosylation. A deeper understanding of COG function and dys- within the Golgi and thereby to severe genetic diseases known as function will likely depend on elucidating its molecular structure. congenital disorders of glycosylation (CDGs) (17, 18). -

Download CGT Exome V2.0
CGT Exome version 2. -

Dosage-Dependent Gynoecium Development and Gene Expression in Brassica Napus-Orychophragmus Violaceus Addition Lines
plants Article Dosage-Dependent Gynoecium Development and Gene Expression in Brassica napus-Orychophragmus violaceus Addition Lines Bowei Cai, Tai Wang, Wenqin Fu, Arrashid Harun , Xianhong Ge * and Zaiyun Li National Key Laboratory of Crop Genetic Improvement, National Center of Oil Crop Improvement (Wuhan), College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; [email protected] (B.C.); [email protected] (T.W.); [email protected] (W.F.); [email protected] (A.H.); [email protected] (Z.L.) * Correspondence: [email protected] Abstract: Distant hybridization usually leads to female sterility of the hybrid but the mechanism behind this is poorly understood. Complete pistil abortion but normal male fertility was shown by one Brassica napus-Orychophragmus violaceus monosomic alien addition line (MA, AACC + 1 IO, 2n = 39) produced previously. To study the effect of a single O. violaceus chromosome addition on pistil development in different genetic backgrounds, hybrids between the MA and B. carinata (BBCC), B. juncea (AABB), and two synthetic hexaploids (AABBCC) were firstly produced in this study which show complete female sterility. A microspore culture was further performed to produce the haploid monosomic alien addition line (HMA, AC + 1 I , 2n = 20) and disomic addition line O (DA, AACC + 2 IO, 2n = 40) together with haploid (H, AC, 2n = 19) and double haploid (DH, Citation: Cai, B.; Wang, T.; Fu, W.; AACC, 2n = 38) plants of B. napus from MA to investigate the dosage effect of the alien O. violaceus Harun, A.; Ge, X.; Li, Z. chromosome on pistil development and gene expression.